- #1
ampakine
- 60
- 0
In this diagram
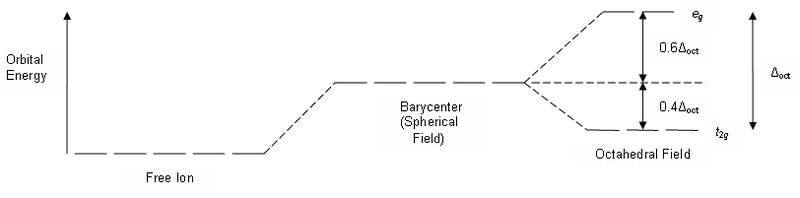
I can't figure out what that barycentre part of the diagram means. The first section of the diagram represents the energy of the d orbitals before the ligands come into the picture and the 3rd section represents the energy of the d orbitals after the 6 ligands have arranged in an octahedral structure. What does the 2nd section of the graph represent?
On one explanation of CFSE they say this:
I can't figure out what that barycentre part of the diagram means. The first section of the diagram represents the energy of the d orbitals before the ligands come into the picture and the 3rd section represents the energy of the d orbitals after the 6 ligands have arranged in an octahedral structure. What does the 2nd section of the graph represent?
On one explanation of CFSE they say this:
but I have no idea what this means. What is the barycentre?If you put an electron into the t2g, like that for Ti3+, then you
stabilize the barycenter of the d orbitals by 0.4 Δo.