- #1
carl
- 8
- 0
--------------------------------------------------------------------------------
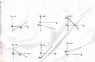
the maximum kinetic energy of a photoelectron, Ek,max , to the smallest amount of energy, E0, needed to free an electron from the metal, and the energy, Eph , of the incident photons of light:
Ek,max = Eph – E0
In addition, you know from Equation 10.1 that Eph = hf where h is the Planck constant and f is the frequency of the incident light.
Which one of the following graphs represents the relationship between Ek,max and f ?
attachment of graphs is attached.
thanks trigger.
Attached Thumbnails
the maximum kinetic energy of a photoelectron, Ek,max , to the smallest amount of energy, E0, needed to free an electron from the metal, and the energy, Eph , of the incident photons of light:
Ek,max = Eph – E0
In addition, you know from Equation 10.1 that Eph = hf where h is the Planck constant and f is the frequency of the incident light.
Which one of the following graphs represents the relationship between Ek,max and f ?
attachment of graphs is attached.
thanks trigger.
Attached Thumbnails