- #1
roam
- 1,271
- 12
I want to know what exactly is the physical significance of absorptance versus absorbance. I have not come across a reference that clearly explains the distinction between the two quantities.
From the definitions:
When light is incident on a material, by finding the fraction that gets reflected and transmitted, we can find the absorptance — the fraction that is absorbed:
$$\underbrace{R}_{\text{reflectance}}+\underbrace{A}_{\text{absorptance}}+\underbrace{T}_{\text{transmittance}}=1$$
Absorbance (also called optical density) follows from the Beer-Lambert law and is written:
$$\text{Absorbance}=\log_{10}\left(1/T\right)$$
For a given sample, I used a spectrophotometer to find both quantities. But the curves appear to give contradicting information about my sample:
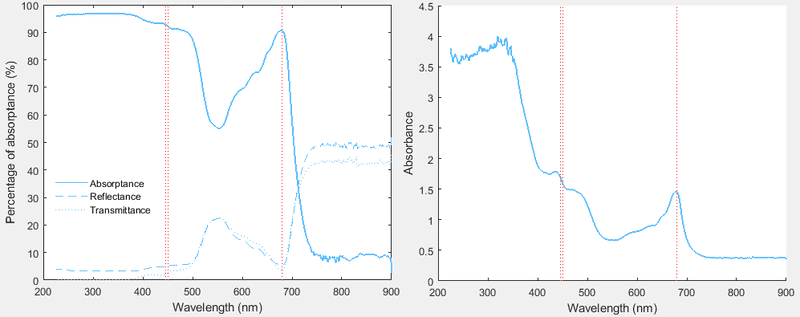
Here is the problem:
The absorptance curve above shows that in the red (~680 nm) and in the blue-violet (<450 nm) regions we have approximately the same level of absorption. But the absorbance curve shows that the absorption is much greater in the blue-violet than in the red. These can't both be true.
I believe that these quantities are providing different information about the sample. But what exactly is the difference?
Any explanation would be greatly appreciated.
From the definitions:
When light is incident on a material, by finding the fraction that gets reflected and transmitted, we can find the absorptance — the fraction that is absorbed:
$$\underbrace{R}_{\text{reflectance}}+\underbrace{A}_{\text{absorptance}}+\underbrace{T}_{\text{transmittance}}=1$$
Absorbance (also called optical density) follows from the Beer-Lambert law and is written:
$$\text{Absorbance}=\log_{10}\left(1/T\right)$$
For a given sample, I used a spectrophotometer to find both quantities. But the curves appear to give contradicting information about my sample:
Here is the problem:
The absorptance curve above shows that in the red (~680 nm) and in the blue-violet (<450 nm) regions we have approximately the same level of absorption. But the absorbance curve shows that the absorption is much greater in the blue-violet than in the red. These can't both be true.
I believe that these quantities are providing different information about the sample. But what exactly is the difference?
Any explanation would be greatly appreciated.