Rayan
- 17
- 1
- Homework Statement
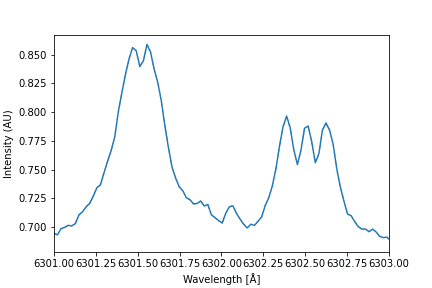
- I'm trying to determine the magnetic field of sunspots using the following graph:
- Relevant Equations
- Formula relating energy and magnetic field:
$$ \Delta E = \mu_B * B $$
But I don't really know how I am supposed to find the energy difference from the graph, how can I know which peaks to use?
Last edited by a moderator: