JD_PM
- 1,125
- 156
Cavitation is a phenomena that occurs in liquids when the local static pressure drops below the vapor pressure; bubbles form within the liquid.
I am studying cavitation in incompressible, two-phase (liquid & gas), homogeneous mixture of cryogenic fluids (liquid nitrogen in particular) within pipes and would like to understand the thermal effects involved. My main sources are: 1) "Implementation and validation of an extended Schnerr-Sauer cavitation model for non-isothermal flows in OpenFOAM" (section 2.3: Thermal model, picture below; it's open source so no issues) and 2) "Method for prediction of pump cavitation performance for various liquids, liquid temperatures, and rotative speeds" (section: Determination of Cavity Pressure Depressions, picture below; it's open source so no issues).
I would like to understand and discuss the thermal models they propose and see if they are applicable to my particular case.
When referring to heat transfer I mean convective heat transfer (which I assume to be a combination of conduction and advection).
1) They state "The convective heat transfer term is subtracted from the latent heat source term". Why? What I understand from this is that latent heat and heat transfer compete but I do not quite get why this should be the case. Let's look at the case of vaporization; the vaporization heat needed for vaporization of a liquid is the convective heat transfer needed to reach vaporization temperature and the latent heat of vaporization of the liquid, and not the difference...
2) They set a heat balance between the heat required for vaporization and the heat drawn from the liquid surrounding the cavity (that is, the liquid-vapor interface).
So I think that they are essentially stating the same idea: the net heat transfer for vaporization is the difference between net latent heat transfer and convective heat transfer.
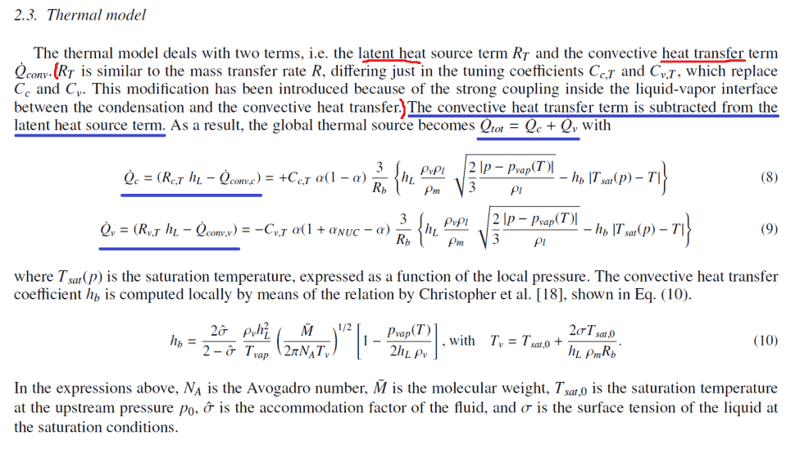
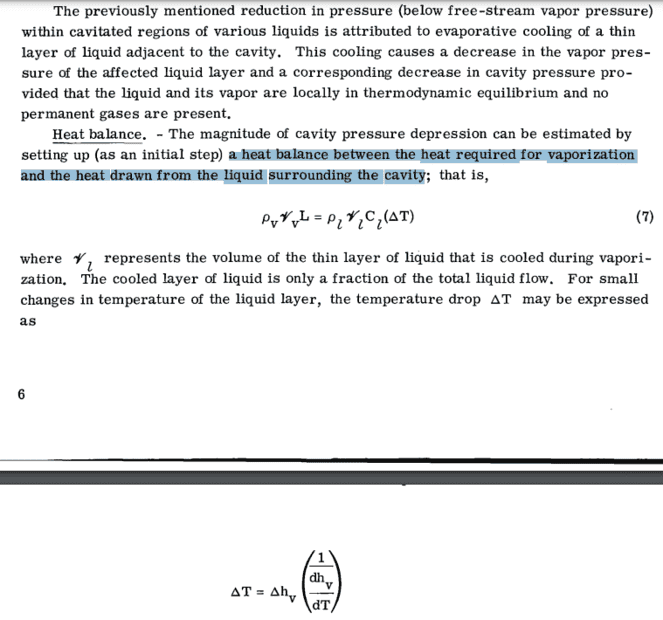
I am studying cavitation in incompressible, two-phase (liquid & gas), homogeneous mixture of cryogenic fluids (liquid nitrogen in particular) within pipes and would like to understand the thermal effects involved. My main sources are: 1) "Implementation and validation of an extended Schnerr-Sauer cavitation model for non-isothermal flows in OpenFOAM" (section 2.3: Thermal model, picture below; it's open source so no issues) and 2) "Method for prediction of pump cavitation performance for various liquids, liquid temperatures, and rotative speeds" (section: Determination of Cavity Pressure Depressions, picture below; it's open source so no issues).
I would like to understand and discuss the thermal models they propose and see if they are applicable to my particular case.
When referring to heat transfer I mean convective heat transfer (which I assume to be a combination of conduction and advection).
1) They state "The convective heat transfer term is subtracted from the latent heat source term". Why? What I understand from this is that latent heat and heat transfer compete but I do not quite get why this should be the case. Let's look at the case of vaporization; the vaporization heat needed for vaporization of a liquid is the convective heat transfer needed to reach vaporization temperature and the latent heat of vaporization of the liquid, and not the difference...
2) They set a heat balance between the heat required for vaporization and the heat drawn from the liquid surrounding the cavity (that is, the liquid-vapor interface).
So I think that they are essentially stating the same idea: the net heat transfer for vaporization is the difference between net latent heat transfer and convective heat transfer.