- #1
nhrock3
- 415
- 0
the standard potential for
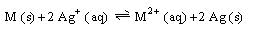
is 1.081
what is the standard potential for M
?
is 1.081
what is the standard potential for M
?