- #1
- 3,759
- 4,199
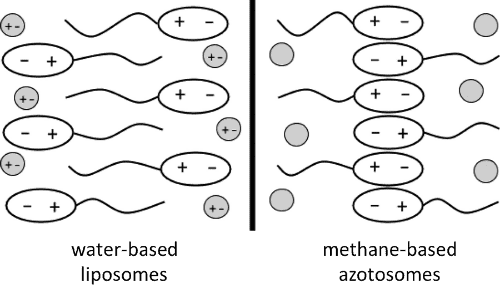
Life on Earth uses water as a solvent, but scientists have long speculated about the possibility of life existing in non-aqueous environments. Titan, Saturn's largest moon, has seas of liquid methane, and scientists at Cornell wanted to test whether structures similar to cell membranes could form on Titan. They took data from the Cassini probe to identify the compounds available on Titan, and performed computer simulations of these molecule to see whether they could form membrane-like structures. They found that one compound in particular, acrylonitrile, form bilayers that are very stable and have flexibilities very similar to cell membranes found on Earth. The researchers termed these structures "azotosomes."
Here's the abstract and citation for the study:
Stevenson, Lunine, and Clancy. (2015) Membrane alternatives in worlds without oxygen: Creation of an azotosome. Science Advances 1: e1400067. doi:10.1126/sciadv.1400067
Of course, membrane-like structures are only one requirement for life, so much work still needs to be done to determine how other aspects of life would work in a cryogenic solution of liquid methane. Furthermore, all of the work in this paper is computational, so the work awaits experimental confirmation that the azotosomes form and function as predicted by the authors' molecular dynamics simulations.
Here's the abstract and citation for the study:
The lipid bilayer membrane, which is the foundation of life on Earth, is not viable outside of biology based on liquid water. This fact has caused astronomers who seek conditions suitable for life to search for exoplanets within the “habitable zone,” the narrow band in which liquid water can exist. However, can cell membranes be created and function at temperatures far below those at which water is a liquid? We take a step toward answering this question by proposing a new type of membrane, composed of small organic nitrogen compounds, that is capable of forming and functioning in liquid methane at cryogenic temperatures. Using molecular simulations, we demonstrate that these membranes in cryogenic solvent have an elasticity equal to that of lipid bilayers in water at room temperature. As a proof of concept, we also demonstrate that stable cryogenic membranes could arise from compounds observed in the atmosphere of Saturn’s moon, Titan, known for the existence of seas of liquid methane on its surface.
Stevenson, Lunine, and Clancy. (2015) Membrane alternatives in worlds without oxygen: Creation of an azotosome. Science Advances 1: e1400067. doi:10.1126/sciadv.1400067
Of course, membrane-like structures are only one requirement for life, so much work still needs to be done to determine how other aspects of life would work in a cryogenic solution of liquid methane. Furthermore, all of the work in this paper is computational, so the work awaits experimental confirmation that the azotosomes form and function as predicted by the authors' molecular dynamics simulations.