- #1
CrimpJiggler
- 149
- 1
I'm trying to get my head around exactly what this ultraviolet catastrophe is. So the early 20th century scientists were studying black body emission spectra (at constant temperatures) and plotted the intensity of the emitted light, against the frequency/wavelength. From what I've read, classical physics theory predicts that the intensity should continue to increase as the frequency of the light increases. Thats the concept I'm stuck on. So here's a black body emission spectrum:
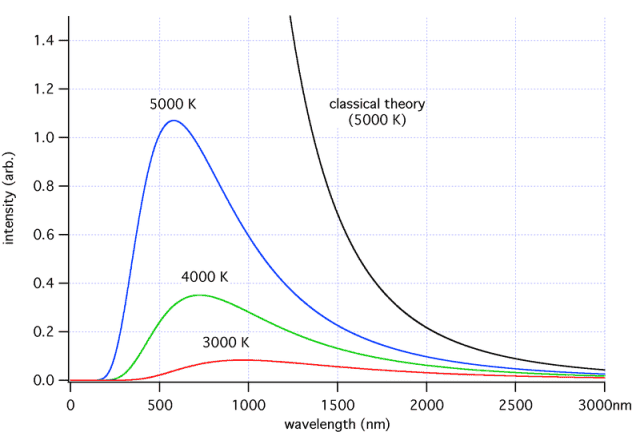
The black line is what classical theory predicts, the other lines are what really happens. Sorry if this is a difficult question to answer but why does classical theory predict that the emitted light should rise in intensity like that?
The black line is what classical theory predicts, the other lines are what really happens. Sorry if this is a difficult question to answer but why does classical theory predict that the emitted light should rise in intensity like that?