- #1
AGNuke
Gold Member
- 455
- 9
I got this problem today in my International Chemistry Olympiad Paper, quite bugging me this question, so I am better off asking it.
My question is, which of the following has R configuration?
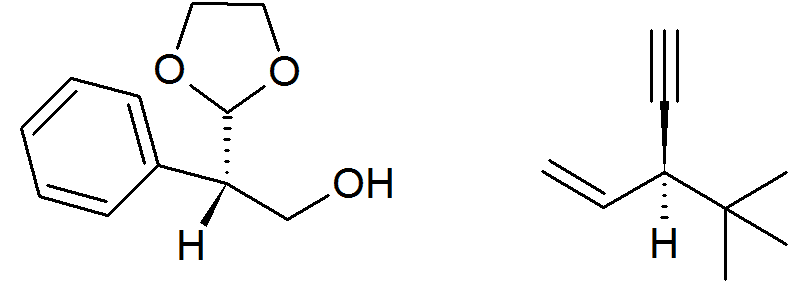
From what I tried, I got both of them as S, and the remaining two options being confirmed S. I got it wrong, but it wouldn't hurt (or it would) to know the right answer.
My question is, which of the following has R configuration?
From what I tried, I got both of them as S, and the remaining two options being confirmed S. I got it wrong, but it wouldn't hurt (or it would) to know the right answer.