- #1
omni
- 192
- 1
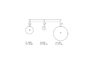
in the picture you can see Three different tanks contain Three different gases.
the temperature are equal to 25 Degrees Celsius in all the Three tanks,
and it not changing.
i asked to calculate the kinetic energy of the Three different gases.
so i thought to use this formula:3/2*RT but i also can use this formula :E=3/2kT but this formula is for average kinetic energy of the Three different gases no?
I'm confused about what formula is correct to solve my question.
thanks.
the temperature are equal to 25 Degrees Celsius in all the Three tanks,
and it not changing.
i asked to calculate the kinetic energy of the Three different gases.
so i thought to use this formula:3/2*RT but i also can use this formula :E=3/2kT but this formula is for average kinetic energy of the Three different gases no?
I'm confused about what formula is correct to solve my question.
thanks.