- #1
fangrz
- 38
- 0
I understand that alanine salt ooks like this:
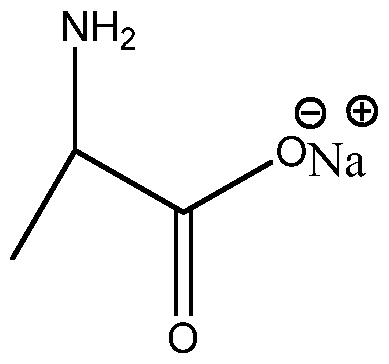
However, does it look like that in solution? Does the salt dissociate? I know that alanine salt has a pH of about 6.113 because it is the average of the 2 pKas. However, if this salt dissociates, it seems that the carboxyl group is a weak base. Does this mean that the amine group is a weak acid? I cannot find online that the amine group goes from NH2 to NH (unless in a protein backbone), but I do see that it can go from NH2 to NH3+?
However, does it look like that in solution? Does the salt dissociate? I know that alanine salt has a pH of about 6.113 because it is the average of the 2 pKas. However, if this salt dissociates, it seems that the carboxyl group is a weak base. Does this mean that the amine group is a weak acid? I cannot find online that the amine group goes from NH2 to NH (unless in a protein backbone), but I do see that it can go from NH2 to NH3+?
 .
.