- #1
Taylor_1989
- 402
- 14
- Homework Statement
- A person living on a planet which has half the radius of the earth, realizes that he can throw a rock 10 times further than he can on earth.
Estimate the relative density of this planet compared to that of earth. Ignore any effect of air resistance.
- Relevant Equations
- $$F=\frac{Gm_1m_2}{d^2}$$
$$\rho=M*V$$
So I am a bit stuck on this question as my result using the above equations dose not give an numerical value which I assume from the question is needed.
So here my method for solving
My first thought was that if on the planet the person can throw a rock 10 time further then that it implies in any direction. So I took the vertical direction, for each planet which is demonstrated by my diagram below.
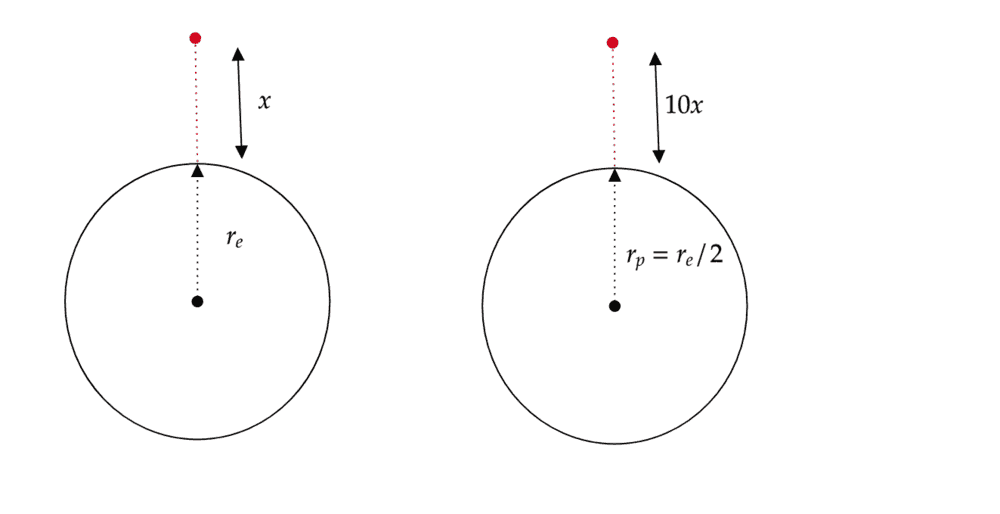
The I used Newtons law of gravity to calculate the gravity on each planet like so.
$$g_e=\frac{GM_e}{d^2}=\frac{GM_e}{(r_e+x)^2}$$$$g_p=\frac{GM_p}{d^2}=\frac{GM_p}{4(r_e+20x)^2}$$
I then rearranged each equation in terms of the mass for each planet.
$$M_e=\frac{g_e(r_e+x)^2}{G}$$
$$M_p=\frac{g_p4(r_p+x)^2}{G}$$
Next I calculated the density of each planet
$$\rho_e=M_e(\frac{4}{3}\pi r_e^3)$$
$$\rho_p=M_p(\frac{1}{6}\pi r_p^3)$$
The by dividing the two density's
$$\frac{\rho_p}{\rho_e}=\frac{M_p}{2M_e}$$
I then sub in my mass equation from earlier and with some manipulation I from the following
$$\frac{\rho_p}{\rho_e}=\frac{g_p(r_e+20x)^2}{8g_e(r_e+x)^2}$$
My issue is, that from the question I believe I am suppose to have some sort of numerical value but can't seem to figure a away of getting one.
Which leaves me with the question
1. What have I assumed wrong in this question, by using the method I have displayed above?
Addtional thought. I was just thinking that if I was on the Earth my distance in x would be x<<$r_e$ and the same goes for the planet 20x<<$r_e$, so could I assumen this in my equation above?
So here my method for solving
My first thought was that if on the planet the person can throw a rock 10 time further then that it implies in any direction. So I took the vertical direction, for each planet which is demonstrated by my diagram below.
The I used Newtons law of gravity to calculate the gravity on each planet like so.
$$g_e=\frac{GM_e}{d^2}=\frac{GM_e}{(r_e+x)^2}$$$$g_p=\frac{GM_p}{d^2}=\frac{GM_p}{4(r_e+20x)^2}$$
I then rearranged each equation in terms of the mass for each planet.
$$M_e=\frac{g_e(r_e+x)^2}{G}$$
$$M_p=\frac{g_p4(r_p+x)^2}{G}$$
Next I calculated the density of each planet
$$\rho_e=M_e(\frac{4}{3}\pi r_e^3)$$
$$\rho_p=M_p(\frac{1}{6}\pi r_p^3)$$
The by dividing the two density's
$$\frac{\rho_p}{\rho_e}=\frac{M_p}{2M_e}$$
I then sub in my mass equation from earlier and with some manipulation I from the following
$$\frac{\rho_p}{\rho_e}=\frac{g_p(r_e+20x)^2}{8g_e(r_e+x)^2}$$
My issue is, that from the question I believe I am suppose to have some sort of numerical value but can't seem to figure a away of getting one.
Which leaves me with the question
1. What have I assumed wrong in this question, by using the method I have displayed above?
Addtional thought. I was just thinking that if I was on the Earth my distance in x would be x<<$r_e$ and the same goes for the planet 20x<<$r_e$, so could I assumen this in my equation above?
Last edited: