- #1
barryj
- 856
- 51
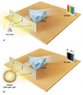
I have been scanning a physics book. See attachment. The diagram shows that a light bulb has a continuous spectrum while the hydrogen has discrete spectral lines. Since the filament in the bulb is tungsten, why doesn't the light bulb also have discrete spectral lines?