- #1
grotiare
- 5
- 4
EDIT: My bad, this is from my professors lecture, but I assume this question is more suitable for the homework section of this forum
The problem gives that pV = constant, and thus is assumed to be an isothermal process. However, the given U1 and U3 are not equal to each other. Is it because it is not specified the gas is ideal that we ignore that ΔU = 0 ?
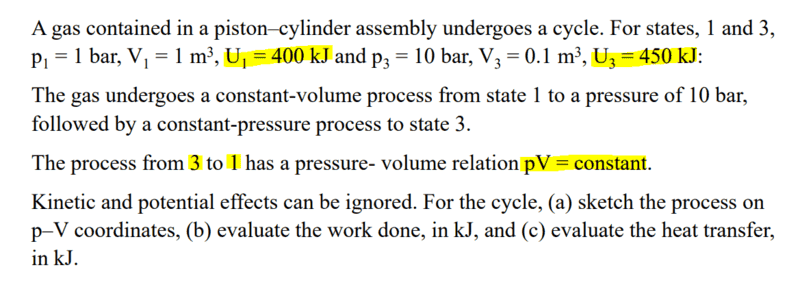
The problem gives that pV = constant, and thus is assumed to be an isothermal process. However, the given U1 and U3 are not equal to each other. Is it because it is not specified the gas is ideal that we ignore that ΔU = 0 ?