- #1
Sho Kano
- 372
- 3
My teacher posted on his website, Lewis structures of different compounds. One of them were N3-
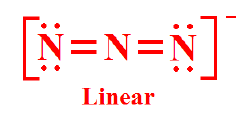
Why isn't there a dipole moment arrow pointing from the central atom to the other Nitrogen? Does the arrow only point from neutral atom to negatively charged atom?
Why isn't there a dipole moment arrow pointing from the central atom to the other Nitrogen? Does the arrow only point from neutral atom to negatively charged atom?