- #36
- 32,820
- 4,720
edguy99 said:To me it is meaningful in a goal to model or simulate processes such as hydration and polymerization where you must track things over time. The model of electrons stuck inside a shell by an amount equal to their ionization energy, together with the normal coulomb force pushing protons apart, leads to the most common forms of hydrogen that are easy to draw and conceptualize (is there a spell checker in this application?).
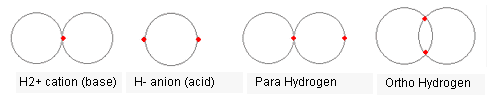
Fine. Let's look at H2 molecule, shall we?
You missed one extremely important aspect of the H2 molecule - the existence of bonding and antibonding state. Look it up. It has quite a bit to do with the phase of the electronic wavefunction. This phase is completely missing in such classical picture of your model, and it means that you have no ability to make a meaningful model of it.
It also indicates that such attempts at producing such a simple picture is inadequate. The fact that we do have a good description of it without needing such simplistic (and incorrect) description is what is puzzling me as far as the need for such erroneous picture. I mean, it is not as if we are just now discovering H atom, and trying to make a model of it to describe it, as what occurred during the early history of quantum mechanics. We already know of a very good (and accurate) description of such systems. Why are we trying to take a large step backwards and produce such toy model when it isn't necessary?
This is very strange!
Zz.