guiromero
- 18
- 0
- Homework Statement
- Butadiene can be react with itself to form a dimer molecule. The reaction is second order in butadiene. The value of the rate constant is 7.7 x 10-3 M-1 s-1.
What is the initial rate consumption of butadiene in a reactor filled with butadiene to a concentration of 3.091 M? Express your answer in M/s.
- Relevant Equations
- r = k*C^2
Hello,
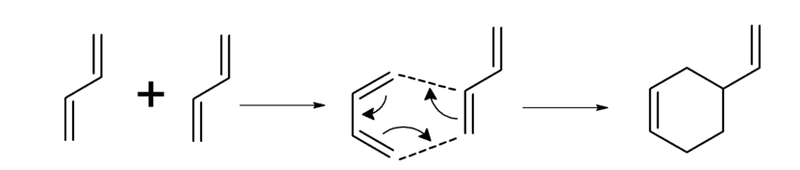
I have a doubt in an exercise about rate of reaction. The statement is quoted above and the reaction scheme is the following:
As the reaction is second order, I tried to apply the formula r = k*C^2.
Where r = rate of reaction
k = rate constant
C = concentration
So, I got:
r = (7.7e-3)*(3,091)^2 = 0,073 M
However, this not correct. I know it because when I click the "Send answer" button, it says "incorrect". Furthermore, the "Show answer" button doesn't display the answer.
Could anyone give some help?
Thanks a lot.
I have a doubt in an exercise about rate of reaction. The statement is quoted above and the reaction scheme is the following:
As the reaction is second order, I tried to apply the formula r = k*C^2.
Where r = rate of reaction
k = rate constant
C = concentration
So, I got:
r = (7.7e-3)*(3,091)^2 = 0,073 M
However, this not correct. I know it because when I click the "Send answer" button, it says "incorrect". Furthermore, the "Show answer" button doesn't display the answer.
Could anyone give some help?
Thanks a lot.