- #1
jackjack
- 3
- 0
I'm just starting an Organic Chemistry course, and I'm completely confused by this problem. It says that the hybridization of nitrogen in C4H9N is sp2, but it has 3 single bonds + 1 lone pair and has a Lewis structure that looks similar to ammonia which is sp3 hybridized. What about pyrrolidine makes the nitrogen sp2?
Just for reference, it looks like this, except there is a double bond between carbons 1 and 2 and carbons 3 and 4 (I think I'm numbering them right ):
):
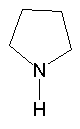
Just for reference, it looks like this, except there is a double bond between carbons 1 and 2 and carbons 3 and 4 (I think I'm numbering them right
 ):
):
Last edited: