- #1
Poorneshwar 2
- 11
- 0
- TL;DR Summary
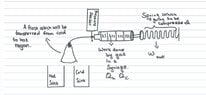
- I have been looking at the heat engine concepts, and i'm a little confused. I have a experimental set-up which has a hot and cold sink, and a syringe where the gas inside expands and compresses when i switch between the sinks, and during the gas expansion, the syringe compresses a spring which compresses a spring. But, in this experiment, only understand that the isothermal processes takes place but how can i include the adiabatic processes so that it actually works like an actual heat engine.
Please, i need help