- #1
Gavroy
- 235
- 0
hi
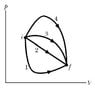
i have this pV-diagram(ideal gas) and i am supposed to say something about the heat, internal energy and work transferred and done on these different paths( i have to bring them in an order like). i do not know how to do this, as this is a pV diagram and these paths are not further specified. i guess that i only need two quantities, as the third quantity is related to the other two by the 1st law of thermodynamics.
i have this pV-diagram(ideal gas) and i am supposed to say something about the heat, internal energy and work transferred and done on these different paths( i have to bring them in an order like). i do not know how to do this, as this is a pV diagram and these paths are not further specified. i guess that i only need two quantities, as the third quantity is related to the other two by the 1st law of thermodynamics.