You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
What is Ice: Definition and 980 Discussions
Ice is water frozen into a solid state. Depending on the presence of impurities such as particles of soil or bubbles of air, it can appear transparent or a more or less opaque bluish-white color.
In the Solar System, ice is abundant and occurs naturally from as close to the Sun as Mercury to as far away as the Oort cloud objects. Beyond the Solar System, it occurs as interstellar ice. It is abundant on Earth's surface – particularly in the polar regions and above the snow line – and, as a common form of precipitation and deposition, plays a key role in Earth's water cycle and climate. It falls as snowflakes and hail or occurs as frost, icicles or ice spikes and aggregates from snow as glaciers and ice sheets.
Ice exhibits at least eighteen phases (packing geometries), depending on temperature and pressure. When water is cooled rapidly (quenching), up to three types of amorphous ice can form depending on its history of pressure and temperature. When cooled slowly, correlated proton tunneling occurs below −253.15 °C (20 K, −423.67 °F) giving rise to macroscopic quantum phenomena. Virtually all ice on Earth's surface and in its atmosphere is of a hexagonal crystalline structure denoted as ice Ih (spoken as "ice one h") with minute traces of cubic ice, denoted as ice Ic and, more recently found, Ice VII inclusions in diamonds. The most common phase transition to ice Ih occurs when liquid water is cooled below 0 °C (273.15 K, 32 °F) at standard atmospheric pressure. It may also be deposited directly by water vapor, as happens in the formation of frost. The transition from ice to water is melting and from ice directly to water vapor is sublimation.
Ice is used in a variety of ways, including for cooling, for winter sports, and ice sculpting.
View More On Wikipedia.org
In the Solar System, ice is abundant and occurs naturally from as close to the Sun as Mercury to as far away as the Oort cloud objects. Beyond the Solar System, it occurs as interstellar ice. It is abundant on Earth's surface – particularly in the polar regions and above the snow line – and, as a common form of precipitation and deposition, plays a key role in Earth's water cycle and climate. It falls as snowflakes and hail or occurs as frost, icicles or ice spikes and aggregates from snow as glaciers and ice sheets.
Ice exhibits at least eighteen phases (packing geometries), depending on temperature and pressure. When water is cooled rapidly (quenching), up to three types of amorphous ice can form depending on its history of pressure and temperature. When cooled slowly, correlated proton tunneling occurs below −253.15 °C (20 K, −423.67 °F) giving rise to macroscopic quantum phenomena. Virtually all ice on Earth's surface and in its atmosphere is of a hexagonal crystalline structure denoted as ice Ih (spoken as "ice one h") with minute traces of cubic ice, denoted as ice Ic and, more recently found, Ice VII inclusions in diamonds. The most common phase transition to ice Ih occurs when liquid water is cooled below 0 °C (273.15 K, 32 °F) at standard atmospheric pressure. It may also be deposited directly by water vapor, as happens in the formation of frost. The transition from ice to water is melting and from ice directly to water vapor is sublimation.
Ice is used in a variety of ways, including for cooling, for winter sports, and ice sculpting.
View More On Wikipedia.org
-
S
Ice cube in an old Micro-oven giving visible electric shocks
I put 1 litre of ice cube to the micro-oven which is age is about 30 years. The shape of the ice cube was a little bit circular. I set the power of the micro to the maximum. There were continuous white electric shocks inside the micro. The ice cube contained a little fish which contained some...- soopo
- Thread
- Replies: 1
- Forum: Electromagnetism
-
G
Heat problem, determine mass of ice cube?
My answer is 12.9 grams, and the correct answer is supposed to be 11.9 grams. I cannot figure out why? Note. the answer i got is 12.9, and not 11.06. I know for this situation, the heat absorbed by the ice equals the heat lost by the water and aluminum, and since there is a phase change from a... -
P
Thermodynamics - again, Part of the ice that melt :S
Homework Statement Stamp of iron the his mass - M and temp' - T0 (T0>100 C deg') has been placed on cube of ice. The mass of the ice - m, the temp' - t0 = 0 C deg' How many present of the ice get melt? Homework Equations Q = mc\Deltat The Attempt at a Solution If I let...- Planck const
- Thread
-
- Tags
- Ice Thermodynamics
- Replies: 1
- Forum: Introductory Physics Homework Help
-
L
How is hail formed in thunderclouds?
Hi Ladies and Gentlemen Watching an episode of Mythbusters a couple of weeks ago, . . it just hit me .. maybe this is how ice is formed in clouds. You have a supercooled bottle of waterbased liquid. You strike a blow to the bottle and watch the liquid turn instanly into ice. Is not it...- LeonStanley
- Thread
-
- Tags
- Ice
- Replies: 7
- Forum: Other Physics Topics
-
C
New to the forum - Stored energy to melt ice
Hi All, New to the forum, but requiring a certain amount of assistance if possible. In relation to ice defrost time, 334 Joules per gram is required to change the state of ice to water and 2.03 joules per gram is required to raise the temperature of ice by one degree? With this in...- chiggs24
- Thread
- Replies: 5
- Forum: Classical Physics
-
J
Bicycle Powered Ice Cream Maker
Hi. I've forgotten some of my basic physics so maybe someone can point me in the right direction. I want to hook up my hand crank ice cream maker to a bicycle. I'm thinking I would have a total of about 5 gears so that I can spread out the work required to crank the ice cream. (I want to crank...- jwjarvis
- Thread
- Replies: 1
- Forum: General Engineering
-

Bottle of supercooled water + agitation = some ice and water
In an experiment plastic bottles of supercooled water were agitated causing visible amounts of ice to form (the water/ice mixture was filtered into a measuring cup, not a large fraction of ice formed). Why did the agitation ("not a lot needed") set off ice formation? Is it the large scale... -
J
Large Ice Mold (taming the beast)
Hello all! It has been some time since I've posted, but I have enjoyed several of the threads I've been following... Anyway, I have a situation with ice, and I'm a comp-sci graduate from way back, not a physics person (wish I was), so I'm looking for help from someone more knowledgeable...- jeffulot42
- Thread
-
- Tags
- Ice
- Replies: 5
- Forum: Other Physics Topics
-
G
If the earth's ice caps melted, how long would a day last?
Homework Statement Suppose that Earth's polar ice caps melted and the water returned to the oceans, so that the oceans were deeper by about 25.7 m. What effect would this have on Earth's rotation? Make an estimate of the resulting change in the length of the day. Homework Equations w=(2pi/T)...- Gold3nlily
- Thread
-
- Tags
- Ice
- Replies: 1
- Forum: Introductory Physics Homework Help
-
J
Length of the day change if polar ice caps melt.
Homework Statement Question requires assumption that the Earth is spherical, all the ice is located at the axis of rotation. Basically if all the ice was to melt uniformally over the surface of the earth, what would be the change in the length of the day? Mass of ice: m= 2.3*10^19 kg Mass of... -
Y
The Science Behind Ice Skating: Debunking the Myth of Pressure and Melting Point
An often encountered but incorrect answer would be that the pressure on the ice lowers its melting point. This doesn't make much sense since this effect can only lower the melting point of ice by a very small amount 1C by a simple calculation in thermal dynamics. So the question is, what...- yileili3
- Thread
-
- Tags
- Ice
- Replies: 4
- Forum: Other Physics Topics
-
Y
Boy Seated On Mound of Ice, Starts Sliding
1. A boy is seated on the top of a hemispherical mound of ice with radius R. He is given a very small push and starts sliding down the ice. At what height does he leave the ice if the ice is frictionless? 2. Conservation of Mechanical Energy: U1 + K1 = U2 + K2; also gravitational energy...- yrsnkd
- Thread
- Replies: 4
- Forum: Introductory Physics Homework Help
-
C
Exploring the Melting of an Ice Cube in Water
Homework Statement an ice cube is melted in water which is continuously stirred to be at a constant temperature of 0 degrees. the stirring is gentle enough so the work done is negligible. my question is why in this case does the heat come from the air to melt the ice cube and not the...- Chronos000
- Thread
- Replies: 1
- Forum: Advanced Physics Homework Help
-
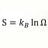
Heat Transfer Coefficient for Ice Melting Time
Homework Statement One liter of water at 30 C ( 30000 calories ) 100 gram (100 cc ) sphere of ice at 0 C is in center of water volume. The ice will absorb 8000 cal melting and final water temp = 22000 cal/ 1100 g = 20 C Assuming mixing and uniform water temp during melting ,and vessel is...- morrobay
- Thread
- Replies: 1
- Forum: Advanced Physics Homework Help
-
S
Advanced ICE Table (chemical equilibria)
Homework Statement Derive an expression for equilibrium concentrations in terms of formality f (and Keq) for the compounds in the the equilibrium reactions shown bellow. Homework Equations A2 + 2S <---> 2SA SA <---> S + A Where S is a substrate (THF) and A is an adduct (BH3)...- sandbeda
- Thread
-
- Tags
- advanced Equilibria Ice Table
- Replies: 6
- Forum: Biology and Chemistry Homework Help
-
P
Enthelpy Change of Ice to Vapor - Show on Graph w/BTU's
Hi!,If someone could help me. I need to show on a graph (line) the change of state of 1 lb of ice, to water. From -35 degrees F to 50 degrees F showing the BTU's required to do so. And where the latent heat and sensible heat come into the graph. I hope someone understands what I'm saying and can...- pipor
- Thread
- Replies: 1
- Forum: Thermodynamics
-
S
Why is Ice More Prevalent in Comets than in Early Planetary States?
I have read a few times that comets are significant transports of ice (perhaps in having supplied water to fill Earth's oceans). That implies that water is more abundant in the few comets (in accumulated mass) that have impacted Earth than that which would have existed within the initial planet...- skeleton
- Thread
- Replies: 2
- Forum: Astronomy and Astrophysics
-
H
Ice poured into water. Final temperature?
I have a kettle of aluminum with the mass of 1,10kg. There's 1,15kg of water in it, with the temperature of 20,2 C. The water is cooled with ice cubes. 150g of ice with a temperature of -25 C are poured in. What is the final temperature? I'm relatively new to physics, and I haven't solved...- hattori_hanzo
- Thread
- Replies: 15
- Forum: Introductory Physics Homework Help
-
C
Optimal Initial Temperature for Melting Ice in Hot Water
Homework Statement Hot water is mixed with an amount of ice having an equal mass to that of the water and an initial temperature of 0 °C. The specific heat capacity of water is 4.2 kJ/(kg K) and the specific latent heat of fusion for ice is 334 kJ/kg. Assume that no heat is lost to the...- chawki
- Thread
- Replies: 27
- Forum: Introductory Physics Homework Help
-
C
Investigating Ice Specific Heat of Fusion
Homework Statement The ice piece of 380 g was taken from the deep-freezer, temperature was -9,6 °C, and well insulated container to the being stone-cold ( 0 °C) water. Inside the Ice piece was small temperature detector. When temperature of the the ice piece had balanced 0 °C. part of the...- chawki
- Thread
-
- Tags
- Fusion Heat Ice Specific Specific heat
- Replies: 18
- Forum: Introductory Physics Homework Help
-
P
Change in internal energy when ice melts to water at 0C
If we melt ice at 0'C to water at 0C, what is the change in the internal energy of the ice-water system? As per the first law of thermodynamics, ΔU = ΔQ +ΔW where they are increase in internal energy, heat flow to the system and work done on the system respectively. If we melt ice at 0C, we...- Pranav Jha
- Thread
- Replies: 18
- Forum: Mechanics
-
O
Investigating an Unexpected Ice Spike Formation
Hi, I was just wondering if anyone could shed some light on how this ice spike formed? All I did was fill a 4" tall, 4" diameter plastic container with water and put it in a normal refridgerator freezer. I came back the next morning and found what you see in the photos. I likely used...- OwtLyur
- Thread
- Replies: 4
- Forum: Classical Physics
-
M
Why Is Dry Ice's Energy Change Considered Internal Rather Than Enthalpy?
Hi everyone I need your help in the following problem: Some dry ice (solid carbon dioxide) at -78oC is placed in a steel jar, which is subsequently sealed. The jar is immersed in a large beaker of water at room temperature until the dry ice has completely vaporized. If the measured energy...- metallica007
- Thread
-
- Tags
- Dry ice Ice Thermodynamics
- Replies: 8
- Forum: Chemistry
-
C
How long it takes for the ice block to hit the ground
Homework Statement 5kg of ice falls away from the edge of the roof of a block of flats, at a height of 26m above the ground. Homework Equations ignoring the air drag, find out: a) how long it takes for the ice block to hit the ground. b) what is the speed at which the ice block hits the...- chawki
- Thread
- Replies: 11
- Forum: Introductory Physics Homework Help
-
N
What is the force exerted by an object on a rope?
Homework Statement Man with mass M pulling with constant horizontal force F along a rope attached to object with mass m. Both the man and the object are on a frictionless surface and separated by distance D. When the man and object meet, what is the velocity of the object? Velocity of the man...- n4rush0
- Thread
-
- Tags
- Ice
- Replies: 2
- Forum: Introductory Physics Homework Help
-
A
Solving for Melting Ice: 500g Lead & -10C Ice Block
Homework Statement a 500 g lead mass is heated to 150 C and placed on a block of ice at -10 C. how much ice, if any will melt? 52 kg = block of ice Homework Equations The Attempt at a Solution i didint know where to start so i did qmcdeltaT + qmcdeltaT = 0, and i failed at it i... -

Water Level Changes in a Beaker with Melting Ice: Exploring Pressure and Density
What happens to the level of water in a beaker when a block of ice floating in it, melts? Does it go up, down or remains same? What is the reason? -
D
How Do You Calculate the Acceleration of a 42kg Ice Block Sliding Down a Slope?
Urgent! Acceleration of an ice block given mass Homework Statement A 42 kg block of ice slides down a slope of 30 degrees. Assuming friction is negligible, what is the acceleration of the block down the incline? Homework Equations Fnet=max W=mg The Attempt at a Solution I have...- dhymas
- Thread
-
- Tags
- Acceleration Block Ice Mass Urgent
- Replies: 1
- Forum: Introductory Physics Homework Help
-
S
Induction Heating Ice: How Does it Work?
Does anybody know how this works? https://www.youtube.com/watch?v=aLwaPP9cxT4- Synetos
- Thread
- Replies: 2
- Forum: Thermodynamics
-
L
Specific Heat of Ice and Copper
Homework Statement A student wants to melt a 200 gram block of ice that is at minus 10.0°C and turn it into liquid water at 15.0°C. The student plans to do this by heating copper pellets to 100.0°C and then dropping them onto the block of ice in a thermally insulated container. How many...- liz_p88
- Thread
-
- Tags
- Copper Heat Ice Specific Specific heat
- Replies: 1
- Forum: Introductory Physics Homework Help
-
M
How Does Water Content in Ice Affect Its Latent Heat of Fusion Value?
We did a calorimeter experiment in my school however the teacher warned us that the latent heat of fusion of ice value will be different from the standard value because the ice has water in it and because the ice melts little before actually doing the experiment. However my question is which way...- mopar969
- Thread
-
- Tags
- Fusion Heat Ice Latent heat
- Replies: 1
- Forum: Introductory Physics Homework Help
-
P
Elastic Collision - Ice Hockey
Homework Statement A HOCKEYPLAYER SKATES AT 7.85 M/S AND HAS A MASS OF 120 KG WHEN HE COLLIDES WITH ANOTHER STAIONARY HOCKEY PLAYER. THE SECOND HOCKEY PLAYER IS NOW MOVING AT 5.67 M/S. FIND THE MASS OF THE SECOND HOCKEY PLAYER AND THE SPEED OF THE FIRST. ASSUME THIS COLLISION IS ELASTIC...- papereater33
- Thread
- Replies: 9
- Forum: Introductory Physics Homework Help
-

Ice Fort Building: From Year 1 to 3
So, my friends and I build ice forts each year (well, not last year... not enough snow). Each year they get a little more ambitious as we apply our previously learned trade-skills to the next generation. I thought I would share a bit here; we just started work on the third generation over this...- FlexGunship
- Thread
- Replies: 17
- Forum: General Discussion
-
R
How much energy does it take to heat a block of ice
Homework Statement How much energy is required to change a 35 g ice cube from ice at −15 C to steam at 117 C? The specific heat of ice is 2090 J/kg * C, the specific heat of water is 4186 J/kg * C, the specific heat of stream is 2010 J/kg * C, the heat of fusion is 3.33 × 10^5 J/kg, and the heat... -
S
Layer of Ice in water pipe (affect on heat transfer)
Hi I am curious to know if anyone can provide a formula/answer to the affect of a thin layer of ice may have on heat loss in water flowing in a pipe. Example. I have a smooth plastic pipe approx 20mm diameter, 5m length. 400ml of water travels down the pipe, approximately 0.5m/s. in -10C...- sam...wise
- Thread
-
- Tags
- Heat Heat transfer Ice Pipe Water
- Replies: 10
- Forum: General Engineering
-
M
Calorimetry problem involving tea and ice
The problem: On a hot summer day, you decide to make some iced tea. First, you brew 1.50 L of hot tea and leave it to steep until it has reached a temperature of T_tea = 75.0 C. You then add 0.975 kg of ice taken from the freezer at a temperature of T_ice = 0 C. By the time the mix reaches...- mcnivvitz
- Thread
-
- Tags
- Calorimetry Ice
- Replies: 4
- Forum: Introductory Physics Homework Help
-
V
Thermal Conductivity Ice Question
Homework Statement Ice has formed on a shallow pond, and a steady state has been reached, with the air above the ice at -9.6°C and the bottom of the pond at 5.5°C. If the total depth of ice + water is 2.1 m, how thick is the ice? (Assume that the thermal conductivities of ice and water are...- VitaX
- Thread
- Replies: 24
- Forum: Introductory Physics Homework Help
-
A
Calculate the mass of ice with heat transfer / latent heat
Homework Statement Find mi, the initial mass of ice. Ti = -20 ci = 2093Jkg-1K-1 Ts = +35 ms = 2kg cs = 837.4Jkg-1K-1 cw = 4187Jkg-1K-1 lf = 3.35x105Jkg-1 S = solid W = water I = ice lf = latent heat of fusion of ice / waterHomework Equations Q=mc\DeltaT Q=C\DeltaT Q=mlThe Attempt at a...- adam640
- Thread
-
- Tags
- Heat Heat transfer Ice Latent heat Mass
- Replies: 4
- Forum: Introductory Physics Homework Help
-
M
Calculating Latent Heat of Ice: A Physics Practical Guide
Homework Statement Hi, I've been given the following information to manipulate the results of my last physics practical: Mass Of Water * Specific Heat Capacity of Water * Change in Temperature of Water = Latent Heat of Ice * Mass of Ice + Mass of Ice * Specific Heat Capacity of Water *...- max0005
- Thread
-
- Tags
- Heat Ice Latent heat
- Replies: 2
- Forum: Introductory Physics Homework Help
-

Why doesn't ice in Saturn's ring sublime away?
Brian Cox on Wonders of the Solar System (episode: Order Out of Chaos) on the Science Channel says the rings of Saturn are made up of chunks of water ice. Water ice? In space? I would expect a chunk of water ice in space would experience a near zero vapor pressure. Wouldn't it? And if...- KenJackson
- Thread
- Replies: 8
- Forum: Astronomy and Astrophysics
-
D
Why is it that my drink tastes better with ice in it?
Like, even if it is ice cold already, it still seems to taste better with ice. I'm guessing it's just a psychological thing?- dratsab
- Thread
-
- Tags
- Ice
- Replies: 9
- Forum: General Discussion
-
D
Melting arctic ice = NMP movement increase?
i was just reading an article i got linked to from google, about the North magnetic poles location is moving towards siberia currently at about 45k per year, which has increased over the past 100 years from around 15k per year, it was explained that it was possibly caused by density variations...- dwaring28720
- Thread
- Replies: 2
- Forum: Earth Sciences
-
M
Strange ice formation, gravity-defying ?
Can anybody explain how this inverted icicle could have come to be formed ? This mug of water had been sitting outside for a few days, last night the temperature in Edinburgh dropped to below zero, and this morning I saw that this ice stalagmite had formed. There was no source of dripping...- murrmac
- Thread
- Replies: 4
- Forum: Other Physics Topics
-
E
Energy required to thaw one gallon of ice
Not sure if the title does this question justice. My question how much energy in KWHs or a fraction thereof, could be saved in refrigerator operation if someone took a gallon of water, let it freeze solid outside during a 0F degree night, then placed it in their refrigerator until it thawed to...- eniwetok
- Thread
- Replies: 6
- Forum: Other Physics Topics
-
L
Calculating Composition & Temp of Mixture After Adding Steam & Ice
Homework Statement One kg of steam at 100 degrees Celsius is added to 1 kg of ice at 0 degrees Celsius. What is the composition and temperature of the resulting mixture. The heat of fusion for water is 79.7 kcal/kg, the heat of vaporization is 539 kcal/kg, and specific heats of ice and water...- lpp208
- Thread
-
- Tags
- Composition Ice Mixture Steam
- Replies: 4
- Forum: Introductory Physics Homework Help
-
K
Temperature change of ice water
A tub of icy water at 0 degrees C hold 100 gallons. You put a 25 pound watermelon with a temperature of 29.4 degrees C. The tub is wrapped in blankets to avoid heat loss. What will be the final temperature of the watermelon, considering that a watermelon is mostly water?- kmarlow123
- Thread
- Replies: 4
- Forum: Introductory Physics Homework Help
-
M
Calculating Buoyant Force of Ice in Water: Homework Practice
1. a)Homework Statement What is the buyant force on 0.90kg of a block of ice floating on liquid water? B) What is the buyant force on 0.90kg of ice held completely submerged under water? Homework Equations Fbuy+mg=0 Fb=mg The Attempt at a SolutionFb=.90kg * 9.18m/s^2=8.8 so i...- mg1977
- Thread
-
- Tags
- Buoyant Buoyant force Force Ice
- Replies: 10
- Forum: Introductory Physics Homework Help
-
O
Steel pulling through ice, find weight of object
Homework Statement Steel object is pulling uniformly through the ice with applied force 2 Newtons. Find object's weight (P), if k of friction is 0.02 Homework Equations F = 2 Newtons k = 0.02 P - ? F = m*a P = m*g The Attempt at a Solution I drow all forces and made this: ma =...- Ockonal
- Thread
- Replies: 8
- Forum: Introductory Physics Homework Help
-
E
Calorimetry: adding ice to water
Homework Statement A 53.5 g ice cube, initially at 0°C, is dropped into a Styrofoam cup containing 369 g of water, initially at 21.4°C. What is the final temperature of the water, if no heat is transferred to the Styrofoam or the surroundings? Homework Equations Lf*m + Mc Delta T = MC Delta...- e46lover
- Thread
-
- Tags
- Calorimetry Ice Water
- Replies: 2
- Forum: Introductory Physics Homework Help
-
A
Finding the final temperature of a final solution. putting ice in soda/water
Homework Statement Suppose you have a 24 ounce mug of soda (treat as water) at room temperature, which is 22 C. Warm soda tastes bad, so you add 125 g of ice at -25 C to the soda. What is the temperature (in C) of the soda once all the ice melts and the solution reaches a uniform temperature...- apbuiii
- Thread
- Replies: 4
- Forum: Biology and Chemistry Homework Help