Concentration Definition and 550 Threads
-
P
Electrochemistry, emf vs HCL concentration
Homework Statement I just conducted a lab for pchem where we took electric potential measurements with a volt meter in a solution for the reaction AgCl(s) +1/2 H2 (g) <--> HCl(aq) + Ag(s) We took the measurements using different concentrations of HCl. We were told that the...- Puchinita5
- Thread
- Concentration Electrochemistry Emf Hcl
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
R
Concentration of Radon in atoms per cubic meter
Homework Statement i) The average concentration of 222Rn in air inside a building is 20Bqm-3; what is the average concentration of Rn in atoms per cubic meter? ii) In a sealed room of volume 50m3 what activity of 238U in a piece of porous rock would eventually give rise to this 222Rn...- Ryomega
- Thread
- Atoms Concentration Cubic Meter Per Radon
- Replies: 2
- Forum: Advanced Physics Homework Help
-
R
How Do You Calculate Weight Percent Concentration of KCl in Water?
Homework Statement What is the weight percent concentration of a saline solution prepared by dissolving 2.440 mole of KCl in 10 L of water? Homework Equations The Attempt at a Solution- rridl001
- Thread
- Concentration Percent Weight
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
J
Chemistry Question Solution Stochiometry And Determining The Concentration?
Homework Statement What is the concentration of all ions after 600.0mL of 2.00 mol/L Barium nitrate is added to 100.0 mL of 2.50 mol/L sodium phosphate. The Attempt at a Solution I first wrote down the equation : 3Ba(No3)2 + 2Na3PO4 = Ba3(PO4)2 (solid) + 6NaNO3 I then found the moles...- justinh8
- Thread
- Chemistry Concentration
- Replies: 2
- Forum: Biology and Chemistry Homework Help
-
S
Solving a Differential Equation: Salt Concentration in a Tank
can you help me ?? A 200 liter tank initially contains 100 liters of water with a salt concentration of 0.1 grams per liter. Water with a salt concentration of 0.5 grams per liter flows into the tank at a rate of 20 liters per minute. Assume that the fluid is mixed instantaneously and that...- sam_0017
- Thread
- Concentration Differential Differential equation Salt Tank
- Replies: 1
- Forum: Calculus and Beyond Homework Help
-
D
Concentration for reactants: KMnO4+Sugar+NaOH
I am given a topic: rate of reaction affected by change in concentration. We have to design a lab - come up with reactions and write a procedure KMnO4+Sugar+NaOH How much KMnO4 should I put in the mixture of Sugar and NaOH. And how much Sugar and NaOH should I start of with I am going to...- dicktoosh
- Thread
- Concentration
- Replies: 3
- Forum: Biology and Chemistry Homework Help
-
M
Finding the concentration in a solution
I am doing a lab to find the concentration of copper in a penny. I am confused on how to find the concentration of copper in each solution I prepared. One solution for example: 2 ml of 0.080 M Cu(NO3)2 add 2 ml of concentrated ammonium hydroxide dilute solution to 50 ml Can someone help...- morgan8222
- Thread
- Concentration
- Replies: 14
- Forum: Biology and Chemistry Homework Help
-
J
What Is the Significance of Using Buffers of the Same Concentration?
"of the same concentration" buffers Homework Statement In the question below what is the signifcance of them saying "of the same concentration" thanks http://www.thestudentroom.co.uk/attachment.php?attachmentid=113982&d=1321805514 Homework Equations The Attempt at a Solution- jsmith613
- Thread
- Buffers Concentration
- Replies: 3
- Forum: Biology and Chemistry Homework Help
-
M
How is weak acid concentration measured?
Is a weak acids concentration determined from when it is in equilibrium, or if it hasn't dissociated at all? i.e. 0.100M CH3COOH. Would this be the concentration of CH3COOH during it's equilibrium: CH3COOH <-|-> CH3COO- + H+ Or just by itself? Thanks! -
P
Differentiate Primary Al & Eutectic Al in Al-17Si Alloys
Hello, I am working on Al-17Si alloys. I have done some castings and in micro-structure I saw formation of Aluminium dendrites. I am unable to find out whether it is primary Al or eutectic Al. One way to find that is to find out Percentage of Si in the dendrites. But how do I do that? Is...- pukb
- Thread
- Analysis Concentration
- Replies: 2
- Forum: Materials and Chemical Engineering
-
M
Solid State Physics- Finding concentration of donor atoms (Nd)
Homework Statement Silicon at T= 300K contains acceptor atoms at a concentration of Na = 5*10^15 cm-3. Donor atoms are added forming an n-type compensated semiconductor such that the fermi level is 0.215 eV below the conduction band edge. What concentration of donor atoms are added...- Matt1234
- Thread
- Atoms Concentration Physics Solid Solid state Solid state physics State
- Replies: 1
- Forum: Advanced Physics Homework Help
-
R
Calculate Concentration of HCl & HOAc for Titration | NaOH & HCl/HOAc Mixture
Hi, I'm in lab in which we have to titrate 0.09M NaOH into 25mL of 0.1M HCl/HOAc mixture. Homework Statement I need help on how to calculate the concentration of HCl and HOAc in the mixture. 0.09M NaOH 1st equivalence point = 14.3mL 2nd equivalence point = 26.9mL Difference of two...- Rissj3
- Thread
- Calculation Concentration Mixture Titration
- Replies: 2
- Forum: Biology and Chemistry Homework Help
-
L
Calculating concentration in molLl^-1 of a solution
Homework Statement Determine the concentration in molL^-1 of a solution of sodium carbonate whoch contains 53g of sodium carbonate in 500ml of solution. Calculate the concentration of sodium and carbonate ions in this solution. Homework Equations no of mols= Mass/Mr mols= [ ] x vol...- leah3000
- Thread
- Concentration
- Replies: 3
- Forum: Biology and Chemistry Homework Help
-
F
Using Ksp to find the concentration
Homework Statement 0.1100 mol AgNO3 translated into 500 mL of a solution containing 0.1000 moles of NaCl and NaBr 0.1000 moles. What are the concentrations of Ag+, Cl-, Br-, after equilibrium has occurred? Solubility product for AgCl: Ksp(AgCl) = 1,82*10^-10 Solubility product for AgBr...- Faka
- Thread
- Concentration Ksp
- Replies: 2
- Forum: Biology and Chemistry Homework Help
-
N
How do you calculate the concentration of nucleotides from DNA?
Hi, I was wondering if given the concentration of DNA, how does one calculate the concentration of nucleotides? Are they the same thing? Thanks. Nancy- nancy189
- Thread
- Concentration
- Replies: 1
- Forum: Biology and Medical
-
Y
How Do Mussels Stabilize Their Shells by Adjusting Seawater pH?
Homework Statement Shells of mussels are mainly of calcium carbonate. IUsually the shell is stable and its content is in equilibrium with the surrounding. Mussels need to build up or reduce their shell, however, for additional protection or during growth. They achieve this by changing the...- yang09
- Thread
- Calculation Concentration Ph
- Replies: 10
- Forum: Biology and Chemistry Homework Help
-
F
Band Bending & Donor Concentration in Semiconductors
When a n-type semiconductor is brought into contact with a metal, then alignment of the chemical potentials of both systems leads to band bending within the depletion layer, e.g on the semiconductor side. does the amount of band bending (change of potential) also depend on the donor...- fk08
- Thread
- Band Band bending Bending Concentration Semiconductors
- Replies: 1
- Forum: Atomic and Condensed Matter
-
S
Change in concentration over time
Homework Statement A tank with a capacity of 400L is full of a mixture of water and chlorine with a concentration of 0.05g of chlorine per liter. The chlorine concentration is to be reduced by pumping in fresh water at the rate of 4L/s. The mixture is kept stirred and pumped out at the rate of...- Scalper
- Thread
- Change Concentration Time
- Replies: 1
- Forum: Calculus and Beyond Homework Help
-

What Is the Concentration of Ions in Different Solutions?
Homework Statement What is the concentration of ____ ions in a ___ mol dm-3 solution of ___?Homework Equations mass/molar mass = number of moles volume/molar volume = number of moles Not sure what other equations... The Attempt at a Solution I'm not understanding how this works. I have...- FeDeX_LaTeX
- Thread
- Chemistry Concentration Ions
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
S
Where to Find Chlorophyll Concentrations for Different Water Types?
Does anyone know of a book, chart, or website with different water types (clear ocean water, great lakes, ...) and their "average" or listed chlorophyll concentrations? Any sources would be greatly appreciated!- swartzism
- Thread
- Concentration
- Replies: 1
- Forum: Biology and Medical
-
K
Schools Selecting Concentration for Grad School
I am a prospective physics student currently attending a 4-year university. As I began to work in a lab in preparation for graduate school, I have been hearing cases of people with physics degree switch their graduate major/concentration and go to physics-related mathematics or engineering...- kitaewolf
- Thread
- Concentration Grad Grad school School
- Replies: 2
- Forum: STEM Academic Advising
-
M
Equilibrium constant and concentration
I am aware that the equilibrium constant, Kc for the reaction {aA}~~+~~{bB}~~~\mathop{\rightleftharpoons}^{k_f}_{k_b}~~~{cC}~~+~~{dD} is \frac{k_f}{k_b} = \frac{[A]^a [B]^b}{[C]^c [D]^d} Now my question is, are the stoichiometric coefficients a,b,c and d, written in simplest whole number...- mishrashubham
- Thread
- Concentration Constant Equilibrium Equilibrium constant
- Replies: 16
- Forum: Chemistry
-
M
Finding the concentration of X
Homework Statement How do I find the Density of X (Part A) and how do I find the Concentration of X in a solution (Part B)? Part A: I have 4mL of X. The mass of 4mL of X is 10g. Calculate the density of X. Part B: I have 7g of X in 100mL of Water. Calculate the concentration of X. The FINAL...- MagicMarker
- Thread
- Concentration
- Replies: 1
- Forum: Precalculus Mathematics Homework Help
-
T
Solving the Mystery of Deriving O2 Concentration in H2/O2 Mixture
In a spherical vessel with a hydrogen H2 and oxygen mixture O2 it is stated in a problem that we can derive that the concentration of oxygen [O2] can be derived to be: P / 3RT. With P the total pressure, R the gas constant en T temperature? How on Earth is this possible? I thought the...- Tigrisje
- Thread
- Concentration deriving Mixture Mystery
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
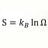
Maximum concentration, molarity, of Carbonic Acid ?
What is the maximum concentration, Moles/liter, possible for one liter of H2CO3 at STP. And how is that calculated ? -
S
Concentration and pH Caculation of Titrations
Homework Statement Calculate the volume of 0.116 M HCl(aq) required to neutralize the following solutions. all the hydroxide ions in 25.0 mL of 0.215 M KOH(aq) 1. What is the molarity of Cl - ions at the stoichiometric point? Homework Equations HCl + KOH --> KCl + H2O The...- Squall
- Thread
- Concentration Ph
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
P
Chemical Equilibrium and Concentration
The reaction between hydrogen and fluorine has an equilibrium constant of 1.15x102 at a certain temperature. Given the reaction equation H2(g) + F2(g) -> 2HF(g), calculate the equilibrium concentration of HF after 3.00 mol of each component is added to a 1.500 L flask. I attempted solving it...- Procrastinate
- Thread
- Chemical Chemical equilibrium Concentration Equilibrium
- Replies: 5
- Forum: Biology and Chemistry Homework Help
-
U
Stress concentration factor using combined static loads
i want to design a machine which is capable of applying all three types of loads that is axial, bending and torsion. Using this application of static loads , I want to measure the stress concentration factor.- usmanyousaf
- Thread
- Concentration Static Stress Stress concentration
- Replies: 1
- Forum: Mechanical Engineering
-
A
Calculation of concentration of a solution.
How does one take into account the fact that volume of a solution increases upon dissolving solute? which volume should be taken as the net volume, the volume of solvent to start with or the full volume of the solution? Suppose I add 20gm of sugar in 100cc (or ml) of water. After sugar...- arroy_0205
- Thread
- Calculation Concentration
- Replies: 5
- Forum: Chemistry
-
G
Schools Grad school help: choosing a concentration
Grad school help: choosing a "concentration" Okay, so I will begin my graduate physics studies this fall. I'm incredibly excited, but very nervous, especially about the comprehensive/qualifying exams, but that's another story... I'm already in my early 30s, married, with a kid. Needless to...- Geezer
- Thread
- Concentration Grad Grad school School
- Replies: 7
- Forum: STEM Academic Advising
-
B
Determining concentration by measuring pressure change
Homework Statement Derive an equation how one can calculate concentration by measuring pressure change in a known volume. Known data: Pressure change dp/dt in a known volume V, volumetric flow rate F, total pressure p and temperature T. Homework Equations Probably ideal gas law...- Boltzano
- Thread
- Change Concentration Measuring Pressure
- Replies: 1
- Forum: Introductory Physics Homework Help
-

How Do Cells Adapt to Changes in Signaling Ligand Concentration?
"In chemical signaling, adaptation enables cells to respond to changes in the concentration of a signaling ligand (rather than to the absolute concentration of the ligand) over a very wide range of ligand concentrations. The general principle is one of a negative feedback that operates with a...- Ahmed Abdullah
- Thread
- Cells Concentration ligand
- Replies: 3
- Forum: Biology and Medical
-
V
How Does Helium Concentration Change with Height in a Cave?
Homework Statement In a large underground cave, the concentration of helium at the base is measured to be 1.00x10^-4. If the air in the cave is at a constant temperature of 10 degrees-C, what is the concentration of helium at the top of the cave, 300m higher? Answer: 1.03 x 10^-4...- v_pino
- Thread
- Concentration Height Temperature
- Replies: 1
- Forum: Advanced Physics Homework Help
-
3
How Do You Calculate Molar Mass from Concentration Data?
Hello! I'm completing a lab for chemistry and would like some help on the last bit figuring out concentration then converting to molar mass. I need to make a data chart with a line of best fit on it with the dependent/independent variables. I understand that concentration is on the X-axis and...- 3riefs
- Thread
- Concentration
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
R
How to Combine Stress Concentrations for Multiple Keyways on a Shaft?
Hello I have done a Soderberg shaft design calculation, taking into account the stress concentration for a keyway. However, I've just noticed that I might need to adapt this for a feather type keyseat, or 2 (parallel, 180deg apart) keyways in the same cross section. I'm sure I read...- robs314
- Thread
- Concentration Shaft Stress Stress concentration
- Replies: 1
- Forum: Mechanical Engineering
-
R
Ka, Kb, calculating concentration of F-
Homework Statement Calculating concentration of F- for .158M of HF? Calculate the equilibrium concentration of F- for .158M of HF in the reaction below.? HF+H20=H30+F- The Kb is 6.66*10-4 Homework Equations Kw=Ka*Kb Ka=[H+][A-]/[HA] The Attempt at a Solution I figured...- ronpaulkid
- Thread
- Concentration
- Replies: 5
- Forum: Biology and Chemistry Homework Help
-
C
Re-arrangement of Stress Concentration Calculation
Hi i was wondering if anyone could help me by showing me how to re-arrange this equation: a=2b*(c\d)½ i need to make c the formula. Any help would be greatly appreciated. Chris- chrisb009
- Thread
- Calculation Concentration Stress Stress concentration
- Replies: 1
- Forum: Mechanical Engineering
-

Electrochemical cells: Is gibbs free energy dependent upon concentration?
Is the gibbs free energy of some reaction in an electrochemical cell dependent upon reactant concentration? The Nernst equation clearly states that open circuit potential is dependent upon concentration, and basic thermodynamics states that open circuit potential is directly proportional to... -
J
Concentration of minority carriers in a pn junction
Homework Statement A semiconductor p-n junction is fabricated where the doping concentration on the p and n sides of the junction is Na=1E18cm^-3 and Nd=1E16cm^-3 respectively. Given that ni=1.18E10cm^-3, T=300k calculate: a) The majority and minority carrier concentrations in the...- jeffy
- Thread
- Concentration Junction Pn junction
- Replies: 2
- Forum: Engineering and Comp Sci Homework Help
-
W
Should I do my MechE concentration in nuclear power or in something else?
I had been planning for awhile to study nuclear engineering at the undergraduate level and eventually do graduate work and enter the field. Now, with what happened recently, I mean is the field shot? I really find this stuff interesting and am wondering if the field still has a good future...- WatermelonPig
- Thread
- Concentration Nuclear Nuclear power Power
- Replies: 5
- Forum: STEM Career Guidance
-
W
Solving Drain Problem: Salt Concentration in Effluent
drain problem? Homework Statement A tank has 1000 m3 of salt solution. The salt concentration is 10 kg/m3. At time zero, salt-free water starts to flow into the tank at a rate of 10 m3/min. Simultaneously salt solution flows out of the tank at 10 m3/min, so that the volume of the solution...- walisyh
- Thread
- Concentration Salt
- Replies: 10
- Forum: Calculus and Beyond Homework Help
-
T
Stoichiometry to find the concentration of O2?
2 Mn2+ + O2 + 4 OH- -> 2 MnO2 + 2 H2O ***** Use the stoichiometry of the chemical reaction to calculate the concentration (mg/L) of O2(aq) and concentration of O2 (aq) (Molarity). DI Water (22 degrees C) Initial Buret Reading: 20.0ml Final Buret Reading: 28.7 ml Volume Na2S2O8 to reach the...- tica86
- Thread
- Concentration Stoichiometry
- Replies: 3
- Forum: Biology and Chemistry Homework Help
-
M
Calculating Chloride Ion Molar Concentration
Homework Statement What is the molar concentration of chloride ions in a solution prepared by mixing 100 ml of 2.0 M KCl with 50 ml of a 1.5 M CaCl2 solution...? I don't know where to start... Homework Equations The Attempt at a Solution- Miike012
- Thread
- Concentration Ion
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
T
Solving the Concentration Gradient: A Calculation Challenge
I have a problem with calculating the concentration gradiant. Here is the question and the solution from the solution manual and the numbers don't add up. A 1-mm sheet of FCC iron is used to contain nitrogen in a heat exchanger at 1200℃. The concentration of N at one surface is 0.04 atomic...- tkuehl
- Thread
- Calculation Challenge Concentration Gradient
- Replies: 1
- Forum: Materials and Chemical Engineering
-
C
Moles...Moles"Calculate Salt & Water Concentration: 3L & 250g NaCl
Homework Statement Calculate the concentration of water and table salt, when 250 grams of table salt NaCl is in 3 litres of liquid. Table salt has a molecular mass of 58,5 g/mol. Homework Equations Give the answer in two decimal precision The Attempt at a Solution What do they mean...- chawki
- Thread
- Concentration Salt Water
- Replies: 2
- Forum: Biology and Chemistry Homework Help
-
E
Solving an Ansys WB Stress Concentration Issue on Stiffener Tip
Ansys WB, getting non-realistic stress conce. (stiffener tip on pipe column face)?? Hi, I am facing a problem in getting accurate stress in Ansys WB for stiffened base plate supporting pipe column (See attached pics) The problem is that I'm getting very high stress concentration at the tip...- EngAB
- Thread
- Ansys Concentration Stress Stress concentration
- Replies: 5
- Forum: General Engineering
-
L
Finding concentration of Permanganate
Homework Statement Calculate the KMnO4 concentration of the solution in the cuvet. The Solution in the covet was made by mixing 5.00 ml of 1.00 M H2SO4 and 1.00 ml of the stock 2.00x10-3 M KMnO4. Homework Equations m1v1=m2v2The Attempt at a Solution I've tried using the dilution formula...- lcary
- Thread
- Concentration
- Replies: 3
- Forum: Biology and Chemistry Homework Help
-
K
Calculating Molality of a Sulfuric Acid Solution
Homework Statement You are given a 77.0% solution of sulfuric acid, H2SO4. What is the molality of this solution? The density of the H2SO4 solution is 1.303 g/mL. Homework Equations The Attempt at a Solution I know I divide 77.0 by 100, and then I have no idea from there.- Ki-nana18
- Thread
- Concentration
- Replies: 2
- Forum: Biology and Chemistry Homework Help
-
M
Semiconductor Carrier Concentration Basics
I have been told that the relationship n.p = (n_i)^2 always holds, where n_i is the intrinsic carrier concentration. This makes sense to me for an un doped semiconductor (sc.). However, for a doped sc., I'm a tad confused. For an n-doped material say, the electron conc. is...- Master J
- Thread
- Basics Carrier Concentration Semiconductor
- Replies: 2
- Forum: Atomic and Condensed Matter
-
K
Calculating Maximum Silver Plating from AgNO3 Solution
Homework Statement Silver nitrate solutions are often used to plate silver onto other metals. What is the maximum amount of silver (in grams) that can be plated out of 4.6 L of an AgNO3 solution containing 3.7% Ag by mass? Assume that the density of the solution is 1.01g/mL . Homework...- Ki-nana18
- Thread
- Concentration
- Replies: 2
- Forum: Biology and Chemistry Homework Help