You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Electrons Definition and 998 Threads
The electron is a subatomic particle, symbol e− or β−, whose electric charge is negative one elementary charge. Electrons belong to the first generation of the lepton particle family, and are generally thought to be elementary particles because they have no known components or substructure. The electron has a mass that is approximately 1/1836 that of the proton. Quantum mechanical properties of the electron include an intrinsic angular momentum (spin) of a half-integer value, expressed in units of the reduced Planck constant, ħ. Being fermions, no two electrons can occupy the same quantum state, in accordance with the Pauli exclusion principle. Like all elementary particles, electrons exhibit properties of both particles and waves: they can collide with other particles and can be diffracted like light. The wave properties of electrons are easier to observe with experiments than those of other particles like neutrons and protons because electrons have a lower mass and hence a longer de Broglie wavelength for a given energy.
Electrons play an essential role in numerous physical phenomena, such as electricity, magnetism, chemistry and thermal conductivity, and they also participate in gravitational, electromagnetic and weak interactions. Since an electron has charge, it has a surrounding electric field, and if that electron is moving relative to an observer, said observer will observe it to generate a magnetic field. Electromagnetic fields produced from other sources will affect the motion of an electron according to the Lorentz force law. Electrons radiate or absorb energy in the form of photons when they are accelerated. Laboratory instruments are capable of trapping individual electrons as well as electron plasma by the use of electromagnetic fields. Special telescopes can detect electron plasma in outer space. Electrons are involved in many applications such as tribology or frictional charging, electrolysis, electrochemistry, battery technologies, electronics, welding, cathode ray tubes, photoelectricity, photovoltaic solar panels, electron microscopes, radiation therapy, lasers, gaseous ionization detectors and particle accelerators.
Interactions involving electrons with other subatomic particles are of interest in fields such as chemistry and nuclear physics. The Coulomb force interaction between the positive protons within atomic nuclei and the negative electrons without, allows the composition of the two known as atoms. Ionization or differences in the proportions of negative electrons versus positive nuclei changes the binding energy of an atomic system. The exchange or sharing of the electrons between two or more atoms is the main cause of chemical bonding. In 1838, British natural philosopher Richard Laming first hypothesized the concept of an indivisible quantity of electric charge to explain the chemical properties of atoms. Irish physicist George Johnstone Stoney named this charge 'electron' in 1891, and J. J. Thomson and his team of British physicists identified it as a particle in 1897 during the cathode ray tube experiment. Electrons can also participate in nuclear reactions, such as nucleosynthesis in stars, where they are known as beta particles. Electrons can be created through beta decay of radioactive isotopes and in high-energy collisions, for instance when cosmic rays enter the atmosphere. The antiparticle of the electron is called the positron; it is identical to the electron except that it carries electrical charge of the opposite sign. When an electron collides with a positron, both particles can be annihilated, producing gamma ray photons.
View More On Wikipedia.org
Electrons play an essential role in numerous physical phenomena, such as electricity, magnetism, chemistry and thermal conductivity, and they also participate in gravitational, electromagnetic and weak interactions. Since an electron has charge, it has a surrounding electric field, and if that electron is moving relative to an observer, said observer will observe it to generate a magnetic field. Electromagnetic fields produced from other sources will affect the motion of an electron according to the Lorentz force law. Electrons radiate or absorb energy in the form of photons when they are accelerated. Laboratory instruments are capable of trapping individual electrons as well as electron plasma by the use of electromagnetic fields. Special telescopes can detect electron plasma in outer space. Electrons are involved in many applications such as tribology or frictional charging, electrolysis, electrochemistry, battery technologies, electronics, welding, cathode ray tubes, photoelectricity, photovoltaic solar panels, electron microscopes, radiation therapy, lasers, gaseous ionization detectors and particle accelerators.
Interactions involving electrons with other subatomic particles are of interest in fields such as chemistry and nuclear physics. The Coulomb force interaction between the positive protons within atomic nuclei and the negative electrons without, allows the composition of the two known as atoms. Ionization or differences in the proportions of negative electrons versus positive nuclei changes the binding energy of an atomic system. The exchange or sharing of the electrons between two or more atoms is the main cause of chemical bonding. In 1838, British natural philosopher Richard Laming first hypothesized the concept of an indivisible quantity of electric charge to explain the chemical properties of atoms. Irish physicist George Johnstone Stoney named this charge 'electron' in 1891, and J. J. Thomson and his team of British physicists identified it as a particle in 1897 during the cathode ray tube experiment. Electrons can also participate in nuclear reactions, such as nucleosynthesis in stars, where they are known as beta particles. Electrons can be created through beta decay of radioactive isotopes and in high-energy collisions, for instance when cosmic rays enter the atmosphere. The antiparticle of the electron is called the positron; it is identical to the electron except that it carries electrical charge of the opposite sign. When an electron collides with a positron, both particles can be annihilated, producing gamma ray photons.
View More On Wikipedia.org
-

Excess Electrons, Milikan Oil Drop
I am writing out the lab for the Milikan Oil Drop experiment and punching out the numbers has shown that my measured charge is off by a factor of 5 from the theoretical amount. theoretical: 1.6e-19 experimental: 3.16e-14 See if I divide this number by 2e5 (which means I am saying there are...- Duderonimous
- Thread
- Replies: 1
- Forum: Introductory Physics Homework Help
-
K
Arranging 3 Identical Electrons
I have seen this question somewhere.. I diidnt get the answer. So please help me. In how many ways three identical electrons can be arranged in two energy levels..?? Is it 2 or 4?? Here will you consider the spin of the electrons??- kashokjayaram
- Thread
-
- Tags
- Electrons
- Replies: 6
- Forum: Quantum Physics
-
O
Electrons move in opposite direction of current?
I was looking at a few circuit diagrams, and it seems like electrons move in the opposite direction as the electric field and current . Why is this? I don't really understand the intuition behind it.- oneplusone
- Thread
- Replies: 4
- Forum: Electromagnetism
-
L
Classical, electrons fall into nucleus, why not planets into sun?
How is electromagnetism different from gravity in that accelerated objects radiate EM waves when accelerated in an electric field but no gravitational waves are generated when objects are accelerated in a gravity field? Why do not planets orbiting the sun generate gravitational waves and... -
M
Does Recoiling Electrons Have 0 Energy When Photon is Scattered 180°?
I'm pretty sure this is a fairly obvious question, but I can't ever be sure.. So, if a photon is "scattered" 180 degrees. Its not being scattered at all, correct? So, then the energy of the "recoiling electrons" would be 0. It makes sense mathematically if I'm doing it right...- MostlyHarmless
- Thread
- Replies: 4
- Forum: Introductory Physics Homework Help
-

HUP and Rest Mass of Electrons
Electrons emit and absorb photons all the time. I heard that each electron is surrounded by a cloud of 10^{20} photons. That suggests to me that the rest mass of an electron must fluctuate, and that raises the prospect of uncertainty. My questions: Is the rest mass of an electron really... -
A
Electrons Collide: What Happens?
What would happen i you forced two Electric currents to collide? Say we have two currents where one current flows "up" the other one "down", and they hit each other at a certain Place?- Antigone
- Thread
-
- Tags
- Electrons
- Replies: 4
- Forum: Electromagnetism
-
A
Current of Electrons: Magnetic Field & Protons
An magnetic field can make a current flow of electrons. But can the magnetic field make the protons move or is it "just" the electrons that flow?- Antigone
- Thread
- Replies: 8
- Forum: Electromagnetism
-

Bounds on the chemical potential of electrons
In a semiconductor, is the chemical potential of electrons limited to take values only between the valence band maximum and conduction band minimum? Are there circumstances where it can cross these bounds?- Useful nucleus
- Thread
- Replies: 4
- Forum: Atomic and Condensed Matter
-
V
Protons Neutrons and Electrons in a neutron star
Hi All, I am preparing for PhD quals and have been looking at problems from other universities. I came across this one and am stumped on how to tackle it. " Assume that the neutron density in a neutron star is 30.1/fm (that is 0.1 neutron per cubic Fermi). Assuming T=0 and ignoring any...- vinceclortho
- Thread
- Replies: 1
- Forum: Atomic and Condensed Matter
-

Understanding X-Ray Emission from Colliding Electrons
i everybody i've studied in my 9th standard that x rays are produced when cathode rays(electrons) hit solid targets such as molybdenum,titanium,etc.. now we'll observe the physics going out there so what's happening there? an electron is coming with some speed say v and hitting the valence... -
Transition of free to bound electrons
Hi, I wonder if there is an elegant way of how to picture (or describe at all) the transition of free electrons (non-quantized, point-like charges) into let's say the bound ground state of the hydrogen atom (in which it becomes quantized and cloud-like). How does the transition occur ? When...- xortdsc
- Thread
-
- Tags
- Bound Electrons Transition
- Replies: 14
- Forum: Quantum Physics
-
W
How to Calculate Current from Electron Flow?
Homework Statement A charge containg 3.7x10^14 electrons flows throug a wire in 150 uS(micro seconds. What is the current? Could some assist me. Is there a shorter way to arrive at the answer? Homework Equations The Attempt at a Solution- W29EMP
- Thread
- Replies: 1
- Forum: Introductory Physics Homework Help
-
N
Do Electrons Lose Heat? Answers to Questions Explained
Do electrons lose heat? If they do, how do they do it? Bremstrahlung for free electrons and going down to a lower energy level for bound electrons? If you had say a metal and cooled it down, would the electrons move slower or just jump to lower energy levels and lose photons?- Northprairieman
- Thread
- Replies: 5
- Forum: Electromagnetism
-
N
Static Electricity and Number of Electrons
Homework Statement When you rub a gold sphere with rabbit's fur, the gold takes on a negative charge (and the rabbit fur positive). Suppose the gold sphere has a mass of 190.0 g and it obtains a net charge of 0.540 μC. Calculate the ratio of the number of electrons added to the gold sphere to...- Nicolaus
- Thread
- Replies: 2
- Forum: Introductory Physics Homework Help
-
G
What happens on un-ionized electrons after ionization?
Hello. I'm now working in the spectroscopy and I'm wondering in one instantaneous moment of ionization. Let's have an atom with multiple bound electrons. The external energy (like in form of photon) is introduced on the atom such that outer bound electron is ionized and the question...- goodphy
- Thread
-
- Tags
- Electrons Ionization
- Replies: 5
- Forum: Quantum Physics
-
S
Flow of electrons hit a potential hole
Homework Statement Flow of 500 electrons per second with kinetic energy 3 eV hits a perpendicular 5 eV potential hole 0.3 nm wide. How many electrons pass per second pass the obstacle? Homework Equations The Attempt at a Solution Hmm, I checked my notes where it is written that... -
J
What causes electrons to move in wave form?
Ok, so I have been searching around the internet for an answer to this and have not found anything besides the fact that electrons move in a quantum way, that is jumping from one place to another without passing through the space between those two places, which I am aware of. So the two main...- John Light
- Thread
- Replies: 11
- Forum: Quantum Physics
-
T
Electrons are negative, same charges repel, then what about this?
Cathode rays are the flow of election, since electrons are -vely charged, is it normal for the rays to travel almost in a bundle without getting dispersed due to it's repulsion? If you say it's because of its velocity, then if you imagine each electrons, they are at almost rest to each other...- Trojan666ru
- Thread
- Replies: 4
- Forum: Electromagnetism
-
Z
X-ray crystallography, protons or electrons?
X-ray crystallography is now 100 years old so this question is probably pretty simple. Is the diffraction coming from the nucleons or the electrons in the sample? Ie. The electrons occupy a more diverse location within the sample (depending on what level/shell they occupy, in fact some may be...- zincshow
- Thread
- Replies: 1
- Forum: Electromagnetism
-
B
Speed of electrons in batteries
i have read around the forum that the speed of electrons in a conductor is somewhat 1mm/s if that is true , then how do galvanic cells operate ? by that rate , about 1000 electrons would come out of the conductor / sec , not only this , but it would seem like the velocity of electrons is...- B4ssHunter
- Thread
- Replies: 14
- Forum: Electromagnetism
-
Field lines of electrons in an atomic orbital
Hello, I have a rather conceptual question I couldn't really find an answer to yet. The electric field lines of an isolated resting electron would simply point from everywhere in space towards the position the electron is located in, with a length inversely proportional to the squared distance...- xortdsc
- Thread
- Replies: 37
- Forum: Electromagnetism
-

Why, IF electrons really orbited atoms, would they lose energy?
Homework Statement Why, IF electrons really orbited atoms, would they lose energy? Homework Equations The Attempt at a Solution I realize they're meant to emit light. But why? I understand vaguely that the E field might be changing with time, since you have a changing magnetic flux... but...- bananabandana
- Thread
- Replies: 4
- Forum: Introductory Physics Homework Help
-
F
Double-slit experiment and watching the electrons
double-slit experiment and "watching the electrons" I'm trying to build a picture of what is happening in the double-hole experiment when you are "watching the electrons" (http://feynmanlectures.caltech.edu/III_01.html#Ch1-S6). It's mostly clear to me but I do have some questions: if you use a...- forcefield
- Thread
- Replies: 33
- Forum: Quantum Physics
-
H
Effective Magnetic Field in Ground-State Hydrogen Atom?
Homework Statement A hydrogen atom in its ground state actually has two possible, closely spaced energy levels because the electron is in the magnetic field B of the proton (the nucleus). Accordingly, an energy is associated with the orientation of the electron's magnetic moment (μ)...- hvthvt
- Thread
- Replies: 1
- Forum: Introductory Physics Homework Help
-

Exploring the Role of Electrons in Polywell Fusion Reactors
Hi , now I was thinking what happens with the electrons that are in the middle of the polywell trapped by the magnetic field coming from the coils? Assuming there is plasma and some fusion takes place at some rate " x" , what actually happens with the electrons in the middle , do they say there...- Crazymechanic
- Thread
-
- Tags
- Electrons
- Replies: 4
- Forum: Electromagnetism
-
C
Radiation - energy distribution of transmitted electrons
Homework Statement What is the meaning of the peaks (for example between 100Kev and 400Kev) in a energy distribution of transmitted electrons graph where the y-axis is probability density (1/eV) and the x-axis is energy (eV) ? When we simulate a source of photons with a given energy...- Cookie97
- Thread
- Replies: 1
- Forum: Advanced Physics Homework Help
-
D
Total charge density of all electrons in the closed subshell n=3, l=2
Homework Statement Hey guys, So the title pretty much says it. I have to find the total charge density produced by all the electrons in a closed subshell where n = 3 and l = 2. The charge density produced by a single electron is (-e)|R_{32}(r)Y_{2,m}(\theta , \phi)|^{2}Homework Equations So he...- Dixanadu
- Thread
- Replies: 1
- Forum: Advanced Physics Homework Help
-

Double-slit experiment with photons vs electrons
Both photons and electrons give the same kind of interference pattern in the double-slit experiment, but while in the case of electrons this carachteristic interference pattern is due to the probabilistic complex wavefunction of the Schrodinger equation within NRQM, for photons no such...- TrickyDicky
- Thread
- Replies: 39
- Forum: Quantum Physics
-
P
Surface plasmons and hot electrons
Hello,Im reading a article about water spliting device in which all necessary charge carriers for water spliting arise from surface plasmons. This is the article: http://www.nature.com/nnano/journal/v8/n4/full/nnano.2013.18.html#f1 So i do not understand how charge carriers arise form...- prehisto
- Thread
- Replies: 5
- Forum: Atomic and Condensed Matter
-
A
Count Valence Electrons for Element: Shell Model
How do you count the number of valence electrons for an element? I mean with the shell model it is pretty obvious but the electrons are actually in orbitals.- aaaa202
- Thread
-
- Tags
- Electrons
- Replies: 4
- Forum: Biology and Chemistry Homework Help
-

What happens to the electrons in a magnet
Suppose I'm holding a magnet and then lift a paperclip(or another magnet) with the magnet. Now the energy density of a magnetic field or the energy that went into making the magnetic field is W = \frac{1} {2 \mu_o} \int B^2 d \tau but since work was done in lifting the magnets, what happenes to...- kmm
- Thread
- Replies: 11
- Forum: Electromagnetism
-
M
What is the Wavelength of the Ejected Electron?
Homework Statement The threshold frequency of a metal is 3.184 x 10^14 Hz. If a photon of wavelength 368 nm strikes the surface of the metal, what is the wavelength of the ejected electron? Homework Equations f (frequency) = c/(wavelength) Eincident = Ethreshold + KE E=fv(wavelength)...- mirandab17
- Thread
-
- Tags
- Electrons Wavelength
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
C
Are All Electrons Created Equal? The Implications of Quantum Field Theory
Were electrons to be slightly different sizes, or have different strengths, or slightly different spin moments, then what effect might this have on the fundamentals of quantum mechanics? So fart, we "assume" an electron has certain characteristics, but are they all the same??- catamongpigeon
- Thread
-
- Tags
- Electrons
- Replies: 40
- Forum: Quantum Physics
-
S
Collision of electrons, kinetic energy?
Homework Statement What is the minimum amount of kinetic energy of electron that hits another electron, so ectra electron-positron pair is produced. Homework Equations ##p^{\mu }=(E/c,\vec{p})## The Attempt at a Solution Before the collision: ##p^{\mu...- skrat
- Thread
- Replies: 5
- Forum: Advanced Physics Homework Help
-
C
Lone Pair Electrons in Dipole Moments
I had to clear this up since there were many threads with the wrong analysis of this problem. Lone pair electrons DO have and affect on dipole moments. Lone pair electrons do have an effect on the net dipole moment of a molecule. A dipole moment is the product of the magnitude of the partial... -
A
Paired electrons in a magnetic field
What would happen if you place a substance containing bonded and paired electrons, such as a biological organ, and place that substance in a homogenous magnetic field? Would the electrons align with the field? I'm a little confused by what an electron pair is. I pretty much know the pauli...- anorred
- Thread
- Replies: 5
- Forum: Quantum Physics
-
A
Spacial distance of paired electrons
Are paired electrons at a spatial distance from one another or are they "mixed" in some way. Why do their magnetic poles cancel?- anorred
- Thread
-
- Tags
- Electrons
- Replies: 2
- Forum: Electromagnetism
-
A
Drude Model: Electrons & Collisions
As far as I understand: In the Drude model we take the electron to be moving in a random direction after each collision (*), such that the mean velocity is simply the average of -eEt/m, which is just -eEτ/m, where τ is the relaxation time. But I am very confused about this basic assumption...- aaaa202
- Thread
- Replies: 3
- Forum: Atomic and Condensed Matter
-
S
Why transition metals can have unpaired electrons in their compounds?
Why can have transition metals unpaired electrons in their compounds? In correlates to their multiple oxidation states, but I still don't know the explanation of it, that would make me satisfied - I suppose it's mathematical, as molecular orbitals are creating. Or is there any explanation?- sludger13
- Thread
- Replies: 5
- Forum: Atomic and Condensed Matter
-

Two paralel streams of electrons.
Consider two parallel streams of electrons in vacuum. Each stream moves with constant velocity and carries a current. According to classical electrodynamics parallel and same direction currents attract each other. The problem is that I can't see how relativistic length contraction can cause...- alpha358
- Thread
-
- Tags
- Electrons
- Replies: 4
- Forum: Special and General Relativity
-
A
Diamagnetic electrons in a homogenous magnetic field
When you place a diamagnetic material in a homogeneous magnetic field, do the electrons of the material align with the field?- anorred
- Thread
- Replies: 5
- Forum: Electromagnetism
-
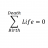
How Many Electrons Are Missing from the Suspended Plastic Sphere?
Homework Statement A small plastic sphere is suspended between two parallel plates like so: http://gyazo.com/0b5425a78b645d85da0b3475d6d71734 How many excess or deficit electrons does the sphere have? Homework Equations ##m=2.60x10^{-15}kg## ##V=265.4V ##...- STEMucator
- Thread
-
- Tags
- Electrons
- Replies: 4
- Forum: Introductory Physics Homework Help
-

Valence electrons in insulators
I was wondering why the valence electrons aren't free to move in insulators? Thanks for any help in advance.- Superposed_Cat
- Thread
-
- Tags
- Electrons Insulators
- Replies: 3
- Forum: Atomic and Condensed Matter
-
Q
Quantum Numbers and Valence Electrons
Homework Statement 1) Apparently it's a true statement that the quantum numbers 2, 0, 0, 1/2 can apply to any of Cl's electrons. But chlorine's electron configuration is [Ne]3s^2 3p^5. What happened to the n = 3 electrons? 2) How many valence electrons can a ground state oxygen atom have...- Qube
- Thread
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
D
Ranking Electric Potential of Electrons in a E Field
Homework Statement Note this is a PRACTICE midterm problem. Not due for homework or for a grade. My exam is on Tuesday "an electron moves from point A to point B in a uniform electric field as show below. Rank the electrons in diagrams 1 through 2 by the changes in potential from greatest...- Dgray101
- Thread
- Replies: 2
- Forum: Introductory Physics Homework Help
-
A
Electromagnetic waves and electrons
Does the electron emit electromagnetic waves?- ahmedelsamra
- Thread
- Replies: 13
- Forum: Chemistry
-
J
Measuring spin on electrons prepared in various directions
Let's say we have 200 electrons. The first batch of 100 of those electrons are prepared in a way that 50% are UP in the x-axis while 50% are DOWN in the x direction. The order is unknown. The second batch of 100 electrons are prepared in the same manner but in the y axis. Bob picks one...- Jeronimus
- Thread
- Replies: 1
- Forum: Quantum Physics
-
Q
Electron configuration, magnetism, and unpaired electrons
My answer key claims it's answer E. I don't think it's right; I think it's cobalt with three unpaired electrons. Not four. A 3d7 configuration, through the Aufbau principle, would fill two of the five d electron pairs completely and leave three half filled. This works mean it's also para...- Qube
- Thread
- Replies: 1
- Forum: Electromagnetism
-
B
Exploring the Relationship between Heat and Electrons: A Scientific Perspective
Hi, I read that heat is the oscillation of atoms. Is it only the nuclei that vibrate or also the electrons? If so, how can they rotate and vibrate? How do electrons react to heat, anyway? If a beam of electrons is rotating in a magnetic field and we heat up the container/the room, how...- bobie
- Thread
- Replies: 12
- Forum: Electromagnetism