Half-life Definition and 130 Threads
-
B
Textbook 'The Physics of Waves': Lifetime of SHM Oscillators
Reference textbook “The Physics of Waves” in MIT website: https://ocw.mit.edu/courses/8-03sc-...es-fall-2016/resources/mit8_03scf16_textbook/ Chapter 2 - Section 2.3.2 [Page 47] (see attached file) Question: In the content, it states that the lifetime of the state in free oscillation is of...- brettng
- Thread
- Half-life Oscillators
- Replies: 5
- Forum: Introductory Physics Homework Help
-

I Radioactivity in quantum physics
In a block of 1 kg of radioactive material that has reached its half-life, are the atoms in a superposed disintegrated/undecayed state (50-50), or are they all individually determined (50% disintegrated and 50% undecayed) before observation ?- Kairos
- Thread
- Atoms Half-life Radioactivity
- Replies: 13
- Forum: Quantum Interpretations and Foundations
-

I Speeding up the half-life of plutonium
I know that it is possible to speed up the half-life of plutonium with neutrons. Who can tell me more about this? Thoughts on harnessing this power to decay plutonium so it's unusable in nuclear weapons?- RobinBanks
- Thread
- Half-life Neutron
- Replies: 5
- Forum: High Energy, Nuclear, Particle Physics
-
I
I Neutrino-Nucleus Interactions and Half-Life Constraints
At what half-life duration, if any, does the likelihood of a neutrino collision within a sample of radionuclide atoms exceed the likelihood of a decay event in those atoms over the same time period? Can they be efficiently excluded by their reaction products, or are they meaningfully difficult...- InkTide
- Thread
- Half-life Neutrinos
- Replies: 5
- Forum: High Energy, Nuclear, Particle Physics
-

I Decay constant of Different Elements and Isotopes?
Different elements and isotopes have different rates of beta decay because the half-life of the element or isotope reflects its stability, which is determined by the nuclear force between the protons and neutrons in the nucleus of the atom. The number of protons and neutrons affects the balance...- Aromalsp
- Thread
- Beta decay Half-life Nuclear force
- Replies: 3
- Forum: High Energy, Nuclear, Particle Physics
-
D
A "Cross-section" and "half-life" of excited states
Hello, I work with a spectrometer that does ionizations through laser 2+1 photons resonant ionization (a high power narrow bandwidth laser is tuned to a precise wavelenght so that it allows reaching an excited energy level of a particular element with the sum of two photons absorbed...- DenisH
- Thread
- Cross-section Excited Excited states Half-life States
- Replies: 3
- Forum: Atomic and Condensed Matter
-
A
I Particle lifetime (half-life) question
If I have a particle with a average lifetime of 15min, if I take 10 particles confined in a box, after 15 min there will be 5 particles. After 15min 2.5 particles and so on... , but so, at the end there will be the last particle that decades. That particle lived far longer than 15min, but is the...- aaronll
- Thread
- Half-life Lifetime Particle
- Replies: 7
- Forum: High Energy, Nuclear, Particle Physics
-
H
Chemistry Calculate the half-life of the antibiotic Ofloxacin in this patient
A 70 kg patient is treated with ciprofloxacin (2 x 500 mg / d) for an infection pulmonary. Ciprofloxacin is an antibiotic eliminated primarily by the kidneys, its apparent volume of distribution is 3 [1 / kg) and its clearance is 0.5 (Wh x kg). Calculate the half-life of ofloxacin in this...- havenly
- Thread
- Half-life
- Replies: 2
- Forum: Biology and Chemistry Homework Help
-
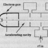
B Shorter half-life and therefore very radioactive -- why?
In reading through The Physics of Energy, the textbook describes the decay chain of U-238: "The longest half-life of any descendent in the chain is less 1 million years. Many half-lives are much shorter, making those nuclides very radioactive." Why does having a short half-life make a...- CPW
- Thread
- Half-life Radioactive
- Replies: 3
- Forum: High Energy, Nuclear, Particle Physics
-
S
I Unbihexium could have a half-life of millions of years?
This article seems to say that the range of estimates go this high. This could be something that would actually stay around long enough to be of some use. https://www.chemistryworld.com/news/beyond-element-118-the-next-row-of-the-periodic-table/9400.article- swampwiz
- Thread
- Half-life Years
- Replies: 4
- Forum: High Energy, Nuclear, Particle Physics
-
S
How Is the Variance Used as Weight in Half-Life Error Analysis?
What is meant by the constant background and how would one deduce the half-life IF not from the fit?- schniefen
- Thread
- Analysis Error Error analysis Half-life
- Replies: 4
- Forum: Calculus and Beyond Homework Help
-
G
What happens if t is greater than half-life?
I tried plugging into the equation t = 3t1/2 and I got A(t) = 0.125A0 since -lambda*t = - ln(2)*t/(t/3) = -ln(2)*3. So I understand that there will be 12.5% left of the original value since three half-lives have passed. But then I realized this won't help me answer the question much. So I'm...- gabriellelee
- Thread
- Half-life
- Replies: 3
- Forum: Introductory Physics Homework Help
-
E
Laser pulses could reduce the half-life of nuclear waste to just 30 minutes
If this could actually be achieved, it would probably win him another Nobel prize, and solve the dilemma of nuclear waste for good. https://bigthink.com/technology-innovation/laser-nuclear-waste?fbclid=IwAR1mFH03XE1x744PHRXCKfWmBt2qeRquOYMAgIXUjqSVEK1kqK2hSkUktcg- ElliotSmith
- Thread
- Half-life Laser Nuclear
- Replies: 9
- Forum: Nuclear Engineering
-
A
What’s the relation between activity and half-life
I’m confused because there are two equations: 1) A=λN 2) A=A0exp^-(λt)If half-life increases, λ decreases, and A decreases according to 1); but, If half life increases, λ decreases, hence exp^-(λt) decreases, A should decreases according to 2)Why is this so? Where went wrong? Thanks!- Angela Liang
- Thread
- Activity Half-life Nuclear physics Relation
- Replies: 2
- Forum: Introductory Physics Homework Help
-

Chernobyl Cesium 137 Half-Life vs. Chernobyl Contamination
Let me start out by saying that I have no idea what I'm talking about. I graduated from Indiana University with a Bachelor's in Spanish, and I work as a Loan Review Specialist at a bank, which has NOTHING to do with my degree, and still yet nothing to do with this topic. But lately, I've become...- Mackenzie Cobb
- Thread
- Cesium Chernobyl Half life Half-life Nuclear Radiation
- Replies: 23
- Forum: Nuclear Engineering
-

Half-life of the "novichok" nerve agent outside of a body?
What is the half-life of "novichok" in, and outside, a biological system? There seems to be an "epidemic" of "novichok" poisoning in Great Britain lately, and given that it's a nerve agent, my understanding is that it should be susceptible to fairly rapid decomposition/breakdown.- Bystander
- Thread
- Body Half-life Outside
- Replies: 5
- Forum: Biology and Medical
-
H
[Poisson Stats] Error on half-life for radioactive decay
Hi there, not sure whether this is in the right section but: I've made two runs of a radioactive decay experiment where I've got a log(N) vs. time plots. From this I've got the decay constants and hence the half-life. I've averaged these two half-lives ( = 160 secs) and now I'm trying to work...- henrym_
- Thread
- Decay Error Half-life Poisson distribution Radioactive Radioactive decay Statistics Stats
- Replies: 1
- Forum: Calculus and Beyond Homework Help
-
C
Finding the Half-Life of a Radioactive Element
Homework Statement In 2 years, 20% of a radioactive element decays. Find its half-life rounded to 2 decimal places. Homework Equations A(t)=a*e^kt The Attempt at a Solution The only thing I've been able to figure out is k=LN(0.2)/2- Conrad Peterson
- Thread
- Half-life
- Replies: 5
- Forum: Precalculus Mathematics Homework Help
-
G
Half-life using Schrodinger's wave equation
Hello guys! I've been learning how to estimate half life using Schrodinger's time-independent wave equation. In class, we divided the energy barrier into five smaller segments just like this webpage http://hyperphysics.phy-astr.gsu.edu/hbase/Nuclear/alpdet.html#c1 I was wondering if we could...- Guessit
- Thread
- Half-life Wave Wave equation
- Replies: 2
- Forum: Advanced Physics Homework Help
-
S
I Fusion half-life of diprotium molecule
A diprotium molecule might undergo tunnelling to fuse by two reactions: p+p→d+e++νe p+p+e→d+νe What is the half-life for the fusion of an isolated diprotium molecule in its ground state (vibrational and rotational)? Naturally big, but also naturally finite. And might be computed. An estimate...- snorkack
- Thread
- Fusion Half-life Molecule
- Replies: 1
- Forum: High Energy, Nuclear, Particle Physics
-
L
How do I use this equation? Estimating half-life of a decay
How should I go about using equation 8.18? Link can be found below. In the book, an example is used where Th-220 --> C-12 + Po-208 with a Q value of 32.1 MeV is used, and it is said to yield t1/2 = 2.3x106 but for the life of me I cannot reproduce this result. This is what I did: Going from...- llatosz
- Thread
- Decay Half life Half-life Nuclear Units
- Replies: 12
- Forum: Advanced Physics Homework Help
-
N
Shouldn't Carbon-14 concentration be the same everywhere?
Why is it that newer organic matter has more carbon-14 than older organic matter? Isn't carbon constantly recycled, because of the carbon cycle? So C14-depleted carbon from old matter would enter the atmosphere, then be breathed in by new organic matter. The new matter would start off with...- Nishantkumar19
- Thread
- Carbon Concentration Half-life Nuclear chemistry
- Replies: 3
- Forum: Chemistry
-
M
Half-Life & Flux: Is Decay Formula Relevant?
Given an initial mass of some isotope subjected to a constant neutron flux, how fast will the mass drop off? Would not the survival curve look exactly like the curve for radioactive decay? Both cases describe a starting mass subjected to a constant transformative force at a rate that depends...- Moniz_not_Ernie
- Thread
- Flux Half-life
- Replies: 5
- Forum: Nuclear Engineering
-

End Amt =Start Amt *e^-0.0391 * t (in years) Half-life =?
I know how to do half life problems and I even have a half-life calculator on my website: http://www.1728.org/halflife.htm However, I cannot calculate half-life when problems are stated in this form: End Amt = Start Amt * e^-0.0391 * t (in years) Half-life =? I know calculus but I find it... -

Time taken for amount of a nuclear material to remain
Homework Statement The half life T½ of Carbon-14 is 5730 years. What is its decay constant? After what length of time will 35% of an initial sample of Carbon-14 remain? Homework Equations Decay constant λ= 0.693 / T½ Where N = amount of radioactive substance, N=N0e-λt The Attempt at a...- InsaneScientist
- Thread
- Half-life Material Nuclear Nuclear decay Time
- Replies: 5
- Forum: Introductory Physics Homework Help
-

Error Propogation for Half-Life
In this homework question we are told to calculate the half-life of an isotope based on count-rates before and after a given time interval, the relevant equation is given below. Half-life = t1/2 = tln(2)/ln (A/A0) The second part asks to determine the standard deviation of the half-life due...- Alejandro Golob
- Thread
- Error Error analysis Half-life Standard deviation
- Replies: 4
- Forum: Introductory Physics Homework Help
-
G
What is the half-life of proton decay in order to detect 3 decays in one year?
Assume you want to find experimental evidence of the proton decay. For this, you use a cylindrical tank with 36 meters high and 17 meters radius which is full of water (18 g / mol). Around the tank are detectors whose overall detection efficiency of a proton decay that gives within the deposit...- Granger
- Thread
- Half-life
- Replies: 2
- Forum: Introductory Physics Homework Help
-
L
Muons hitting Earth half-life explanation in SR
Muons time dilation from the Earth's frame (and length contraction of Earth's atmosphere from muon's frame) is the usual explanation of the fact that muons reach the Earth when they shouldn't just by their rest half-life. My question is if the explanation based on differential aging(different...- loislane
- Thread
- Earth Explanation Half-life Muons Sr
- Replies: 12
- Forum: Special and General Relativity
-
J
Partial Half-Life of 22Na: Ec & β+ Emission
Homework Statement What are the partial half of 22Na for decay by a)Ec b) β+ emission Homework Equations λ=ln2/T1/2 The Attempt at a Solution this what I do T1/2 =2.602 Yr λ=ln2/2.602 λ=0.266 yr-1what is the difference between a)Ec b) β+ emission there is no Percentage of each decay type.!- jije1112
- Thread
- Half-life Nuclear Nuclear chemistry Nuclear physics Partial
- Replies: 1
- Forum: Advanced Physics Homework Help
-

Do hadron particles have half-life?
Hi, i heard that as the universe will expand and the energy will grow sparse, in the end the matter itself will turn into radiation that will loose energy. So i wanted to ask, can a proton decay? and is it the same phenomenon as radioactive decay? Does it mean that even hydrogen (with no...- danihel
- Thread
- Hadron Half-life Particles
- Replies: 8
- Forum: High Energy, Nuclear, Particle Physics
-
U
Why doesn't Rubidium decay to Strontium?
Both ##^{87}_{37}Rb## and ##^{87}_{38}Sr## are odd-even nuclei, so we can ignore the pairing term ##\delta##. I tried to calculate the most stable Z for a given A by finding ##\frac{\partial B}{\partial Z} = 0##. That gives the most Z-stable value of ##Z_0 = \frac{2\gamma A}{4\gamma + \epsilon...- unscientific
- Thread
- Beta decay Decay Half-life Nuclear physics
- Replies: 6
- Forum: High Energy, Nuclear, Particle Physics
-

What is the meaning of unbound in the context of half-lives of nuclei?
I have been looking at half-life of various isotopes and many of the tables / information I am finding is giving energies (MeV, KeV) for certain half lifes. This does not make sense to me, am I missing something ? For example : http://ie.lbl.gov/toi/listnuc.asp?sql=&A1=5&A2=5 5Li and 5He give...- Mark lamorey
- Thread
- Half-life Isotopes
- Replies: 10
- Forum: Other Physics Topics
-
S
Estimating Half-Life of Alpha Decay: Nucleus ##^{252}_{98}Cf##
Homework Statement Nucleus ##^{252}_{98}Cf## alpha decays with half life time ##t_{1/2}=2.6## years. What is the velocity of the alpha particle after the decay? Estimate the half life time of the nucleus after the decay. Homework EquationsThe Attempt at a Solution Ok, no worries about the...- skrat
- Thread
- Alpha Alpha decay Decay Half-life Nucleus
- Replies: 2
- Forum: Advanced Physics Homework Help
-

Is half-life inversely related to the radiation dosage of an element?
Is half-life inversely related to the radiation dosage of an element? That is, if an element has a longer half-life is it safer? If so, why is plutonium so dangerous, even though it has a very long half life- Calpalned
- Thread
- Element Half-life Radiation
- Replies: 7
- Forum: Nuclear Engineering
-
A
Factors which influence to half-life speed
Looking for researches, articles and experimental values conserning factors which influence to half-life speed for different isotopes.- ansenko
- Thread
- Factors Half-life Speed
- Replies: 10
- Forum: High Energy, Nuclear, Particle Physics
-
M
Uncovering Half-Life Data for Odd Isotopes: A Search for Fission Products
The Sigma database at Brookhaven lists seven isotopes as fission products for which I can't find half-life data. I've tried nea6287-JEFF-20-1, the NuDat_2 web site, Nuclear Wallet Cards and Wikipedia. Anybody have any other ideas? The isotopes are 74-As-m1 85-Se-m1 86-Br-m1 109-Ru-m1...- Moniz_not_Ernie
- Thread
- Half-life Isotope
- Replies: 6
- Forum: Nuclear Engineering
-
T
Is There a Shortcut for Solving Half-Life Decay Equations?
A 100 mg sample of magnesium-27 decays by 7% of its previous mass every minute. Determine its half-life and start the half-life decay equation. The textbook that I got this from (Nelson Physics 11) tells me the answer, but uses a long and annoying process to find it: creating a table at...- Trustworthy
- Thread
- Decay Half-life
- Replies: 5
- Forum: High Energy, Nuclear, Particle Physics
-

Half-Life: Definition, Equations, and Extended Explanation
[SIZE="4"]Definition/Summary The half-life, t_{1/2}, of an inverse exponential process (an exponential decay) is the time taken for the amount to reduce by one-half. It is constant. Processes with a half-life include radioactive decay, first-order chemical reactions, and current flowing...- Greg Bernhardt
- Thread
- Half-life
- Replies: 1
- Forum: General Math
-
M
What does a radionuclide with a short half-life mean?
I don't understand what this means? Including artificially produced nuclides, more than 3300 nuclides are known (including ~3000 radionuclides), many of which (> ~2400) with decay half-lives shorter than 60 minutes. This list expands as new radionuclides with very short half-lives are...- marc32123
- Thread
- Half-life
- Replies: 11
- Forum: Other Physics Topics
-
K
How long does it take for the activity of the radionuclide to decrease by 50%?
Homework Statement The activity (decays per second) of a certain radionuclide is observed to decrease by 87.5% in 30 hours. What is the half-life of the radionuclide? Homework Equations (1) A(t) = A_{0}e^{-λt} (2) T(1/2) = 0.693/λ The Attempt at a Solution I'm just stuck on...- kirstlen
- Thread
- Half-life
- Replies: 10
- Forum: Introductory Physics Homework Help
-
J
MHB Half-Life of Radioactive Waste: 150,000 Years
After 150 thousand years, only 1/8 of the original amount of a particular radioactive waste will remain. The half-life of this radioactive waste is how many thousand years? 150,000 years / 1/8 150,000 / .125 = 1,200,000 If this isn't correct is the 1/8 suppose to be stay as a fraction, turned...- Joystar77
- Thread
- Half-life
- Replies: 4
- Forum: General Math
-
P
Half-Life Formula: Is It Possible?
Hi, I have always wondered, is there a known formula that predicts the half life of an atom based on known values (e.g., number of protons and neutrons) rather than observation?- pantheid
- Thread
- Formula Half-life
- Replies: 2
- Forum: High Energy, Nuclear, Particle Physics
-
D
Deriving Error Equations for Half-Life Calculations
Homework Statement How to derive an error equation: t1/ 2 = ln 2/λ= 0.693/λ. Confused, and don't even know where to start. 2. The attempt at a solution σ(t1/2)= σ(ln2)/(ln2) + σ(λ)/λ- dab353
- Thread
- Derivative Error Half-life
- Replies: 2
- Forum: Introductory Physics Homework Help
-
H
Is this half-life answer kosher?
Question: U has a very long half life and decays through a series of daughter products ending with a stable isotope of lead. Very old samples of U ore, which have not undergone physical or chemical changes, would be expected to show an equilibrium between the daughter elements provided that...- henpen
- Thread
- Half-life
- Replies: 5
- Forum: Introductory Physics Homework Help
-
M
What is the next step after finding the k-value in half-life calculations?
Maybe this is a dumb question... Let's say I want to figure out the age of a rock and I have the half life of an element. If I have this equation y(t)=e^{kt} where first I figure out the k-value using the half life. That part I get. Now that I have the k-value I 're-use' the formula...- mateomy
- Thread
- Calculations Half-life
- Replies: 4
- Forum: Introductory Physics Homework Help
-
G
Proving the half-life of Potassium-40
Homework Statement "K-40... decays by two radioactive processes. It can decay by electron capture or β- emission. It is found that a sample containing 4.0x10^18 nuclei of K-40 emits a total of 68 β- particles and photons each second. This shows the half life is 1.3x10^9 years." Use the...- GandhiReborn
- Thread
- Half-life
- Replies: 2
- Forum: Introductory Physics Homework Help
-

Finding Mass of Isotope After Radioactive Decay by Half-Life
Homework Statement A radioactive sample contains 2.25g of an isotope with a half-life of 3.8 days. How much of the isotope in grams will remain after 11.0 days? Homework Equations The Attempt at a Solution Hi! I've just started college this semester. I'm taking Introductory Chemistry. Right...- rakeru
- Thread
- Decay Half-life Isotope Mass Radioactive Radioactive decay
- Replies: 4
- Forum: Biology and Chemistry Homework Help
-
M
Hello,I'm revising half-life for GCSE and have come across some
Hello, I'm revising half-life for GCSE and have come across some questions regarding fractions. For example, "what fraction of the original nuclei will still be unstable after 5 half-lives?" Am I right in thinking it would be 1/20 as 1/2 x 5 = 1/20 or is that too simple? Thanks, Molly- Molly1235
- Thread
- Gcse Half-life
- Replies: 3
- Forum: Introductory Physics Homework Help
-
D
Fotran program to find half-life of a radioactive material
Homework Statement I need to write a program that, given the decay constant of a radioactive material, will calculate numerically (to withing one second) the time taken for half of the orginal sample of material to decay.Homework Equations λ = decay constant C0 = start amount of material...- Daniel1992
- Thread
- Half-life Material Program Radioactive
- Replies: 5
- Forum: Engineering and Comp Sci Homework Help
-
D
Half-life decomposition, find final concentration
Homework Statement In a first order decomposition in which the rate constant is 0.03 sec-1, how much of the compound (in mol/L) is left after 39 sec, if there was 2.00 mol/L at the start? I'm using a few equations and trying to plug it in but I don't know whether they are appropriate or not...- dolpho
- Thread
- Concentration Decomposition Final Half-life
- Replies: 5
- Forum: Biology and Chemistry Homework Help