Hydrogen Definition and 1000 Threads
-
A
Powerline electrolysis hydrogen production
Hydrogen production consumes a lot of energy because one is running current through water in order to produce hydrogen. Why can't we use current that already runs in powerlines for example like HVDC and create a series electrolysis apparatus. Water electrolysis needs a minimum voltage...- artis
- Thread
- Electrolysis Hydrogen
- Replies: 11
- Forum: Electrical Engineering
-

Zero-point energy of diatomic hydrogen (particle in a box)
If we take ##H_2## as a "particle" in a box, can the zero-point energy of the overall molecule be calculated as the sum of the zero-point energies of all particles in ##H_2##? That is $$E_ {1,H_2}=\frac{2h^2}{8m_{\mathrm{H^+}}L^2} + \frac{2h^2}{8m_{\mathrm{e^-}}L^2}=...- Mayhem
- Thread
- Box Energy Hydrogen Zero-point energy
- Replies: 3
- Forum: Advanced Physics Homework Help
-
M
I Escape of Hydrogen from the Earth's Atmosphere
Hi ! I ask you two questions: 1- If hydrogen escapes from the Earth's atmosphere as it happens, because if there are anaerobic bacteria that produce hydrogen naturally among some other living beings, because among the thousands and millions of years that life has been on earth, hydrogen was...- MartinG
- Thread
- Atmosphere Escape Hydrogen
- Replies: 15
- Forum: Classical Physics
-

Mining waste for cheaper hydrogen fuel production
https://phys.org/news/2021-09-ingredient-cheaper-hydrogen-fuel-production.html -
T
A Relation of ionisation voltage vs. pressure for hydrogen
Suppose we have specific amount of hydrogen gas enclosed inside a metallic chamber and that is connected to a very high positive voltage source. As the voltage is positive and that's so high that all the molecules inside the chamber lost their electrons and there is nothing but a nuclei gas is...- T C
- Thread
- Hydrogen Pressure Relation Voltage
- Replies: 31
- Forum: Other Physics Topics
-
H
Overlap integral of hydrogen molecule
Hi! Some help with this problem would be much appreciated. The overlap integral is defined as ##S = \int \phi_A (\mathbf{r}_A) \phi_B (\mathbf{r}_B) \,d\mathbf{r}##. For the two orbitals, I have that $$\phi_A = \frac{1}{\sqrt{\pi}} \Big( \frac{1}{a_0} \Big)^{3/2} e^{-r_A / a_0}$$ for the 1s...- hicetnunc
- Thread
- Hydrogen Integral Molecule Overlap
- Replies: 1
- Forum: Advanced Physics Homework Help
-
M
I Dirac equation in the hydrogen atom
Hello! I went over a calculation of the hydrogen wavefunction using Dirac equation (this one) and I am a bit confused by the angular part. The final result for the wavefunction based on that derivation is this: $$ \begin{pmatrix} if(r) Y_{j l_A}^{m_j} \\ -g(r)...- Malamala
- Thread
- Atom Dirac Dirac equation Hydrogen Hydrogen atom
- Replies: 1
- Forum: Quantum Physics
-

Optimizing Hydrogen Combustion Catalysts for Efficient Stove Design
I am interested in build a hydrogen stove for cooking and wish to use a catalytic stove. I cannot find much information about this anywhere, but only that nickel can be used for hydrogen rather than platinum. 1) Can I just use stainless scotch brite scrub pad as catalyst assuming it...- seandepagnier
- Thread
- Catalyst Combustion Hydrogen
- Replies: 1
- Forum: Chemistry
-
H
Discrepancies in Electron Wavefunction Probability Calculation?
Hi. I would love if someone could check my solution since me and the answer sheet I found online don't agree. The probability is given by the triple integral \begin{align*} \int_0^{r_b} \int_0^{2\pi} \int_0^\pi |\psi (r)|^2 r^2 \sin{\theta} \,d\theta \,d\phi \,dr &= \frac{1}{\pi...- hicetnunc
- Thread
- Electron Hydrogen Wavefunction
- Replies: 7
- Forum: Advanced Physics Homework Help
-

I More info on HT/HD shielding constants?
I was wondering how they measured or calculated these differences? I don't know what they refer to, but assume theyre scattering Hydrogen with Tritium or Deuterium to measure the difference of something. https://physics.nist.gov/cgi-bin/cuu/Results?search_for=shielding+difference- skywalker
- Thread
- Constants Deuterium Hydrogen Shielding Tritium
- Replies: 1
- Forum: High Energy, Nuclear, Particle Physics
-

I Rotation curve with neutral hydrogen and dark matter
Flat rotation curve in galaxies is determined by observing neutral hydrogen which is co-distributed with dark matter. What is the rotation curve profile of neutral hydrogen in galaxies where there is less dark matter?- Ranku
- Thread
- Curve Dark matter Hydrogen Matter Neutral Rotation
- Replies: 7
- Forum: Astronomy and Astrophysics
-
O
I General solution of the hydrogen atom Schrödinger equation
Hello everyone! I have two questions which had bothered me for quite some time. I am sorry if they are rather trivial. The first is about the general solution of the hydrogen atom schrödinger-equation: We learned in our quantum mechanics class that the general solution of every quantum system...- Oliver321
- Thread
- Atom Atomic physics General General solution Hydrogen Hydrogen atom Molecular physics Quantum mechahnics Schrödinger Schrodinger equation
- Replies: 9
- Forum: Quantum Physics
-
P
B Can a hydrogen atom become a neutron?
I read this from Nasa's website: "Within the first second after the Big Bang, the temperature had fallen considerably, but was still very hot - about 100 billion Kelvin (1011 K). At this temperature, protons, electrons and neutrons had formed, but they moved with too much energy to form atoms...- ProjectFringe
- Thread
- Atom Hydrogen Hydrogen atom Neutron
- Replies: 5
- Forum: High Energy, Nuclear, Particle Physics
-
H
Calculating Pressure & Mass for Hydrogen Tank Filling
As I stated in the summary I'd like to calculate the required pressure and mass of a tank of hydrogen that would allow me to fill a second tank with 10kg of H2 at 700bar. I am trying to get an idea of the feasibility of doing this, putting aside, for now, how the primary tank would itself be...- H2Fill
- Thread
- Hydrogen Mass Pressure Tank
- Replies: 7
- Forum: General Engineering
-
J
Hydrogen in the Aviation Industry -- Survey
Hello Everyone- I'm completing my Master's Degree in Commercial Aviation. I'm conducting a capstone research project about the potential of an industry shift to hydrogen, and have created a quick survey. If you have additional insight in addition to the survey, please respond in the comments...- jrob11
- Thread
- Aviation Hydrogen Industry Survey
- Replies: 1
- Forum: Aerospace Engineering
-

Verify the average value of (1/r) for a 1s electron in the Hydrogen atom
For spherical coordinate, ##dV = r^{2} \sin {\theta} dr d\theta d\phi## Therefore, $$<\frac{1}{r}> = \frac{1}{(\pi)(a_0)^{3}} \int_{0}^{\infty} {r e^{\frac{-2r}{a_0}} dr \int_0^{\pi} \sin {\theta}} d\theta \int_0^{2\pi} d\phi$$ From partial integral, I've found: $$\int_{0}^{\infty} r...- agnimusayoti
- Thread
- Atom Average Average value Electron Hydrogen Hydrogen atom Value
- Replies: 6
- Forum: Advanced Physics Homework Help
-
L
B Nuclear fusion question -- Calculations for Hydrogen fusing into Helium
I read in 2 books that 4 atoms of Hydrogen fuse and give 1 atom of Helium and 2 electrons, and these 2 electrons convert to light. And that the mass of the Helium is less than the mass of the 4 atoms of Hydrogen, thus that the mass lost converted to light too. But I sum up the masses of...- luckis11
- Thread
- Calculations Fusion Helium Hydrogen Nuclear Nuclear fusion
- Replies: 20
- Forum: High Energy, Nuclear, Particle Physics
-

I Solving Schrödinger's equation for a hydrogen atom with Euler's method
Hi, first-time poster here I'm a student at HS-level in DK, who has decided to write my annual large scale assignment on Schrödinger's equation. My teacher has only given us a brief introduction to the equation and has tasked us to solve it numerically with Euler's method for the hydrogen atom...- Tuca
- Thread
- Atom Euler's method Hydrogen Hydrogen atom Method Quantum Schrödinger Schrodinger equation Schrodinger's equation
- Replies: 2
- Forum: Quantum Physics
-
D
Spherically symmetric states in the hydrogen atom
The equation $$\frac{\hbar^2}{2m}\frac{d^2u}{dr^2}-\frac{Ze^2}{r}u=Eu$$ gives the schrodinger equation for the spherically symmetric functions ##u=r\psi## for a hydrogen-like atom. In this equation, substitute an assumed solution of the form ##u(r)=(Ar+Br^2)e^{-br}## and hence find the values...- docnet
- Thread
- Atom Hydrogen Hydrogen atom States Symmetric
- Replies: 2
- Forum: Advanced Physics Homework Help
-
E
A Feynman solution for the radial wave function of the hydrogen atom
Reading the classical Feynman lectures, I encounter the formula(19.53) that gives the radial component of the wave function: $$ F_{n,l}(\rho)=\frac{e^{-\alpha\rho}}{\rho}\sum_{k=l+1}^n a_k \rho^k $$ that, for ##n=l+1## becomes $$ F_{n,l}=\frac{e^{-\rho/n}}{\rho}a_n\rho^n $$ To find ##a_n## I...- emilionovati
- Thread
- Atom Feynman Function Hydrogen Hydrogen atom Radial Wave Wave function
- Replies: 4
- Forum: Quantum Physics
-
L
I Hydrogen atom: Energies and eigenstates
When we say energy levels of the hydrogen atom. Are that energies of the atom or of an electron in the atom? Also corresponding states? http://hyperphysics.phy-astr.gsu.edu/hbase/quantum/hydwf.html Why energies are negative? E_n \propto \frac{-1}{n^2}- LagrangeEuler
- Thread
- Atom Eigenstates Energies Hydrogen Hydrogen atom
- Replies: 17
- Forum: Quantum Physics
-
T
1420 MHz--- the emission frequency of cold hydrogen gas
I recently finished reading Paul Davies book The Eerie Silence, which is a book about the SETI (Search for ExtraTerrestrial Intelligence) project. In The Eerie Silence, Davies says that scientists using radio telescopes to search for radio messages from space aliens set their radio telescopes...- timmeister37
- Thread
- Cold Emission Frequency Gas Hydrogen
- Replies: 30
- Forum: Chemistry
-

I Why does a hydrogen gas tube produce a hydrogen atomic spectrum?
To measure the atomic hydrogen spectrum people often uses hydrogen gas tubes as light source. Since the gas in the tube is the molecule ##H_2## , why we obtain the spectrum of atomic hydrogen? My guess is that because the voltage is so high, so that the molecules are totally dissociated. If...- amilton
- Thread
- Atomic Atomic physics Gas Hydrogen Spectrocopy Spectrum Tube
- Replies: 9
- Forum: Atomic and Condensed Matter
-

Relative volume of combusted hydrogen
Hi, If I had a volume of Brown's gas at 20°C / 1atm, what would the expected volume [of the resultant steam] be immediately after it was ignited? Thanks! Bob -
P
Chemistry Why is HCl called Hydrogen Chloride (by IUPAC naming)?
Hydrogen Monochloride- pkc111
- Thread
- Hcl Hydrogen Iupac
- Replies: 9
- Forum: Biology and Chemistry Homework Help
-

An experiment for the determination of hydrogen ionization energy
hi guys i saw this experiment in an old book that uses the gas vacuum tube "thyratron" for determining the hydrogen ionization energy , the idea i guess is straight forward : we set the filament current to a specific value then the electrons starts to emit from the cathode traveling its way to...- patric44
- Thread
- Determination Energy Experiment Hydrogen Ionization Ionization energy
- Replies: 1
- Forum: Introductory Physics Homework Help
-
B
Hydrogen Fusion Engine: Steel Sphere & Sulfuric Acid
Have a thick steel hollow sphere with a inside radius of 20 cm. Then fill it up with sulfuric acid and add water. Then remove the oxygen ions. then give the sphere a negative charge and all the hydrogen ions move to the surface of the inside sphere. then charge sphere positive and the hydrogen...- Bruce Haawkins
- Thread
- Engine Fusion Hydrogen
- Replies: 5
- Forum: Nuclear Engineering
-
Z
What is the Saha equation and how does it apply to hydrogen gas?
I have no clue. I am stuck. I would appreciate If someone could help me out. I just know I need to use the Saha equation.- zenodv
- Thread
- Gas Hydrogen
- Replies: 1
- Forum: Introductory Physics Homework Help
-

I Can Dynamical Systems Explain Electron Behavior in Hydrogen Atoms?
Hello,everyone. Can dynamical system be used to describe the behavior of the electron in Hydrogen atom?- thaiqi
- Thread
- Atom Dynamics Electron Hydrogen Hydrogen atom
- Replies: 7
- Forum: Quantum Physics
-

I Q re a photon ionizing a hydrogen atom
This question is a followup to another thread. https://www.physicsforums.com/threads/qs-re-the-behavior-of-atoms-after-decoupling-completed.994581/ I would like to explore the issue raised by @kimbyd. . . . after reionization the temperature of the intergalactic medium is dominated by...- Buzz Bloom
- Thread
- Atom Hydrogen Hydrogen atom Photon
- Replies: 1
- Forum: Quantum Physics
-

A strange wave function of the Hydrogen atom
I am trying to solve the following exercise. In a H atom the electron is in the state described by the wave function in spherical coordinates: \psi (r, \theta, \phi) = e^{i \phi}e^{-(r/a)^2(1- \mu\ cos^2\ \theta)} With a and \mu positive real parameters. Tell what are the possible values...- omegax241
- Thread
- Atom Function Hydrogen Hydrogen atom Quantum measurement Strange Wave Wave function Wavefunction
- Replies: 5
- Forum: Advanced Physics Homework Help
-

I What is the density of hydrogen atoms in the Universe?
UNITS m is meters kg is kiliograms K is degrees Kelvin s is seconds J is joules u is daltons = 1.66053906660(50)×10−27 kg https://en.wikipedia.org/wiki/Dalton_(unit) 1 pc = 3.085678 x 1016 m CONSTANTS MH = mass of hydrogen atom = 1.007825 u = 1.673532784796145 ×10−27 kg...- Buzz Bloom
- Thread
- Atoms Density Error Hydrogen Universe
- Replies: 3
- Forum: Cosmology
-

Normalization of the wave function for the electron in a hydrogen atom
- jjson775
- Thread
- Atom Electron Function Hydrogen Hydrogen atom Normalization Wave Wave function
- Replies: 23
- Forum: Introductory Physics Homework Help
-
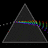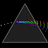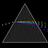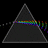
Hydrogen Emission Spectrum and Electron Energy Levels
1. The 4th line from the left, being the aqua blue line, corresponds to a wavelength of 486 nm, as blue light has a wavelength in the range 450-495 nm. 2. This is where I am having the most difficulty, I have tried to answer the question comprehensively but I am not satisfied with my answer. In...- AN630078
- Thread
- Electron Emission Emission spectrum Energy Energy levels Hydrogen Levels Spectrum
- Replies: 2
- Forum: Introductory Physics Homework Help
-

Elastic Collision of Hydrogen and Carbon Atoms
1.p=mv Before the collision: p hydrogen = 1.7x10^-27 * 500 =8.5*10^-25 kg ms^-1 p carbon = 2.0x10^-26 * 0 = 0 kg ms^-1 p total before = 8.5*10^-25 kg ms^-1 The sum of momentum prior to the collision is equal to the total momentum after a collision, momentum is constant, therefore; p before = p...- AN630078
- Thread
- Atoms Carbon Collision Elastic Elastic collision Hydrogen
- Replies: 9
- Forum: Introductory Physics Homework Help
-

Doppler effect and hydrogen alpha distributions
https://www.asi.edu.au/wp-content/uploads/2015/03/PhysicsASOE2014solutions.pdf q 14b) i) Assuming that the planet is rotating at a constant rate, shouldn't the distribution be even across all wavelengths, or do I have something very wrong with my model. I take the graph as the summation of...- aspodkfpo
- Thread
- Alpha Distributions Doppler Doppler effect Hydrogen
- Replies: 3
- Forum: Introductory Physics Homework Help
-

Reaction of Potassium Permanganate and Hydrogen Peroxide solution
With the peroxide solution in excess, we added 10ml vinegar and 10ml of different concentrations of permanganate and timed the reaction. The basics are that the more concentrated the permanganate , the faster the reaction. I would just like to know what the theoretical relationship is between...- neilparker62
- Thread
- Hydrogen Hydrogen peroxide Reaction
- Replies: 8
- Forum: Chemistry
-

I Effects of hydrogen anions in the solar atmosphere
What is the population density of ##H^-## hydrogen anions in the Sun's atmosphere, how stable are they, what limits their numbers, and since they are the dominant source of opacity in radiative transfer in stellar atmospheres, what do they effectively "hide"?- sciFax
- Thread
- Atmosphere Effects Hydrogen Solar
- Replies: 4
- Forum: Astronomy and Astrophysics
-

B Hydrogen anions - how do they form?
Having read a few Wiki articles on and around the subject, I am now somewhat aware of the means by which these ##H^-## anions acquire the extra electrons "donated" by e.g. ionized alkali atoms in stellar atmospheres, and the means by which they provide opacity. What I don't understand is how it...- sciFax
- Thread
- Form Hydrogen
- Replies: 21
- Forum: Atomic and Condensed Matter
-

Help with 3-D interactive QM visualisation of a hydrogen atom
First of all, I got to decide what I'm going to use to make the simulation. I know Fortran, Matlab etc but I'm pretty sure these won't help me much. I learned some C++ a couple years ago but my knowledge is rusty, however I think I'm going to use that combined with Unreal Engine, since it makes...- AndreasC
- Thread
- Atom Hydrogen Hydrogen atom Qm Visualisation
- Replies: 5
- Forum: Computing and Technology
-
G
B The Coronal Heating Problem - The Hydrogen Fusion Core disappears?
In previous manifestations explaining the Coronal Heating Problem, scientists pointed out the discontinuity between a very hot Solar interior, the 'cool' Photosphere and a Hot Corona. According to the newly modified entry in Wikipedia, they now explain the discontinuity only between a cool...- Gfellow
- Thread
- Core Fusion Heating Hydrogen
- Replies: 18
- Forum: Astronomy and Astrophysics
-
A
B Area of a Circle in an Electron's Hydrogen Atom
My textbook says "A is the area of the circle enclosed by the current" (produced by an electron in a hydrogen atom), A = ##\pi r^2 \sin(\theta)^2##. I don't understand where the ##\sin(\theta)^2## comes from.- Anthill
- Thread
- Area Atom Circle Hydrogen Hydrogen atom Surface area
- Replies: 1
- Forum: General Math
-
K
TISE solution for a hydrogen atom
I am unable to complete the first part of the question. After I plug in the function for psi into the differential equation I am stuck: $$\frac {d \psi (r)}{dr} = -\frac 1 a_0 \psi (r), \frac d{dr} \biggl(r^2 \frac {d\psi (r)}{dr} \biggr) = -\frac 1 {a_0}\frac d {dr} \bigl[r^2 \psi(r) \bigr] =...- Kynsuo
- Thread
- Atom Differential equation Hydrogen Hydrogen atom Quantum phyics Schrodinger equation
- Replies: 22
- Forum: Introductory Physics Homework Help
-

I need a help on thermodynamics physics relating to Metallic Hydrogen
- Haozi02133
- Thread
- Fermi energy Hydrogen Physics Thermodynamic Thermodynamics
- Replies: 1
- Forum: Advanced Physics Homework Help
-

Quantum Mechanics Hydrogen Atom Expectation Value Problem
I can not solve this problem: However, I have a similar problem with proper solution: Can you please guide me to solve my question? I am not being able to relate Y R (from first question) and U (from second question), and solve the question at the top above...- cemtu
- Thread
- Angular momemtum Atom Expectation Expectation value Expected value Hydrogen Hydrogen atom Mechanics Quantum Quantum mechanics Value
- Replies: 24
- Forum: Advanced Physics Homework Help
-

Quantum Mechanics hydrogen atom eigenfunction problem
This is a general property of eigenvectors of Hermitian operators. State functions are a particular class of vector, and it is easiest to work in the general formalism (I am hoping to show how ket notation makes qm easier, not just do standard bookwork at this level). Suppose O is a Hermitian...- cemtu
- Thread
- Atom Eigenfunction Hydrogen Hydrogen atom Mechanics Quantum Quantum mechanics
- Replies: 1
- Forum: Advanced Physics Homework Help
-
Y
Is it possible to ionize hydrogen peroxide at home?
Hi, I wonder maybe anyone can help me. I’m trying to ionize hydrogen peroxide mist with the help of a high voltage electrical filed / corona arc. And I wonder if there is a way to know if I ionize the hydrogen peroxide mist at all? And if so, how much do I ionize it (compering to deferent...- yair252525
- Thread
- Hydrogen Hydrogen peroxide
- Replies: 11
- Forum: Chemistry
-
B
Is hydrogen peroxide effective against the corona virus?
If oxygen is effective against bacteria virus and cancer why isn't it used against coved-19? DMSO is known to bond with certain substances and could carry HP into the blood stream and kill off COVID-19.- bill hart
- Thread
- Corona Coronavirus Covid-19 Hydrogen Hydrogen peroxide Virus
- Replies: 1
- Forum: Biology and Medical
-
G
I Perturbation lines for the 1s hydrogen electron
Does anyone know theory about how the perturbation lines are for 1s hydrogen electron? By perturbation I mean the perturbation that is caused by moving an electron so that the E-field lines it emits becomes dragged. by perturbation I mean for example dragging a charge as described below Above...- georg gill
- Thread
- Electron Hydrogen Lines Perturbation
- Replies: 10
- Forum: Quantum Physics
-
B
Radius of the electron orbit in a Hydrogen atom
I am really stuck on what to do here in this question I have arrived at forming an equation to work out the radius of electron orbit from doing the following However I do not know what to do next as I don't know what the value of n (quantum number) must be? :oldconfused: Any help would be...- Bolter
- Thread
- Atom Electron Hydrogen Hydrogen atom Orbit Radius
- Replies: 15
- Forum: Introductory Physics Homework Help