Internal Definition and 957 Threads
-
N
Internal Energy and Temperature Changes When Mechanical Work is Done on a System
Homework Statement What happens to the internal energy of a system and its temperature when mechanical work is done on it? Homework Equations ΔU=Q-W The Attempt at a Solution First Law of Thermodynamics say:the change in internal energy of the system is equal to the heat added to the...- nafo man
- Thread
- Energy Internal Internal energy System
- Replies: 5
- Forum: Advanced Physics Homework Help
-
L
When does the change in enthelpy=change in internal energy?
Sometimes in my book, a problem justifies ΔU=ΔH for a process, such as combustion in a bomb calorimeter, by saying that since the number of moles of gas doesn't change, they are equal. In other questions, the number of moles doesn't change (such as an irreversible expansion of a perfect gas)...- LogicX
- Thread
- Change Energy Internal Internal energy
- Replies: 1
- Forum: Thermodynamics
-
A
Internal Conversion of Bi-207/Pb-207
Hi, I am aware that due to the three body kinematics of beta decay, the energy spectrum of the electrons emitted is a continuous spectrum (where at one extreme the electron gets all of the disintegration energy and the antineutrino none, and the converse at the other extreme) I am doing an...- atay5510
- Thread
- Internal
- Replies: 1
- Forum: High Energy, Nuclear, Particle Physics
-
I
Internal Energy and Work Done in an Adiabatic Process
Homework Statement The question is as following: http://i56.tinypic.com/se547s.png Homework Equations W = F x distance or p d(v) . Potential energy : mgh , k.e : 1/2mv^2 The Attempt at a Solution Well I am stuck on part c, now I am aware that in adiabatic process Internal...- ibysaiyan
- Thread
- Adiabatic Adiabatic process Energy Internal Internal energy Process Work Work done
- Replies: 17
- Forum: Advanced Physics Homework Help
-
D
Thermodynamics: internal energy change
Homework Statement Enthalpy of formation of a mole of atomic hydrogen = 218kJ. Enthalpy changes when a mole of atomic hydrogen is formed by dissociating half a mole of molecular hydrogen. Calculate ΔU of the process of molecular hydrogen dissociation. Homework Equations ΔH = ΔU + Δ(PV)...- Dr. Science
- Thread
- Change Energy Energy change Internal Internal energy Thermodynamics
- Replies: 8
- Forum: Introductory Physics Homework Help
-
J
Internal energy when stirring a liquid.
Homework Statement A liquid is irregularly stirred in a well-insulated container and thereby undergoes a rise in temperature. Regard the liquid as the system. (a) Has heat been transferred? How can you tell? (b) Has work been done? How can you tell? Why is it important that the stirring is...- JustinLiang
- Thread
- Energy Internal Internal energy Liquid
- Replies: 5
- Forum: Introductory Physics Homework Help
-
S
Batteries and Internal resistance
A 6.0 V battery has an internal resistance r. The measured voltage (emf of the battery) is 6.0 V. When connected to a resistor R the terminal voltage is 5.9 V and the current is 0.18 A. I managed to get a) What is the internal resistance r of the battery? 0.556 ohm as well as b) What is...- sabak22
- Thread
- Batteries Internal Internal resistance Resistance
- Replies: 14
- Forum: Engineering and Comp Sci Homework Help
-
A
Internal resistance of cells in parallel
I know, as a matter of fact, that the equivalent resistance of three identical cells in parallel is equal to the internal resistance devided by 3. When we are concerning simply 3 equal resistors in parallel, I know that the outcome resistance is what. But when it comes to three cells with...- abcd8989
- Thread
- Cells Internal Internal resistance Parallel Resistance
- Replies: 1
- Forum: Electromagnetism
-
V
Optics: Total internal reflection problem
Homework Statement A lamp is placed in the center at the bottom of a 2m deep swimming pool. The lamp emits light in all directions. Starting from a point directly above the lamp, a man in a canoe paddles until he no longer can see the lamp. How far did he paddle the canoe? Assume that the...- viny
- Thread
- Internal Optics Reflection Total internal reflection
- Replies: 1
- Forum: Introductory Physics Homework Help
-
M
DIHEDRAL GROUP - Internal Direct Product
I have to prove that D4 cannot be the internal direct product of two of its proper subgroups.Please help.- mehtamonica
- Thread
- Dihedral Direct product Group Internal Product
- Replies: 6
- Forum: Linear and Abstract Algebra
-
L
Difference between enthelpy and internal energy.
U=q+w U is the total kinetic and potential energy of the system. H=U+PV Enthalpy is the internal energy of the system, plus how much energy it takes to set up the system. I don't understand how the PV term from enthalpy is not included in the internal energy. It seems like it would...- LogicX
- Thread
- Difference Energy Internal Internal energy
- Replies: 11
- Forum: Thermodynamics
-
R
Structural Analysis Problem- internal hinge
Homework Statement The Attempt at a Solution for part 1: i figured that this structure is not in static equilibrium. once load "P" is applied, the roller at "D" will function as a fulcrum and ABC will lift (make an angle with the horizontal). is that assumption correct...- ride5150
- Thread
- Analysis Hinge Internal Structural Structural analysis
- Replies: 1
- Forum: Engineering and Comp Sci Homework Help
-
L
Difference between Temperature and Internal Energy
Hi :) Uhh, this question is quite simple but I'm still confused between these two terms... Am I right to say that internal energy is the sum of kinetic energy and potential energy of all the particles in a system? Meaning the two factors are the number of particles, and the energy of each...- lokifenrir96
- Thread
- Difference Energy Internal Internal energy Temperature
- Replies: 14
- Forum: Thermodynamics
-
P
P.Chem1: Internal energy in isothermal, reversible reactions
I have these two homework problems, as well as solutions. What I do not understand is why the solution for one is not the solution for the other. First problem: A sample consisting of 1.00 mol Ar is expanded isothermally at 0 deg Celc from 22.4 dm3 to 44.8 dm3 reversibly. Calculate q, w, delta...- Puchinita5
- Thread
- Energy Internal Internal energy Isothermal Reactions Reversible
- Replies: 4
- Forum: Biology and Chemistry Homework Help
-
L
Trouble Working Out Internal Forces in Truss - Need Help!
hi all, im having trouble working out the internal forces in this question, i tried before and got stuck working out the forces at joint C. i have been to see my tutor however he is on holiday till after the deadline for this assignment. handy... i was working out the forces by the...- liamh05
- Thread
- Forces Internal Internal forces Truss
- Replies: 2
- Forum: Engineering and Comp Sci Homework Help
-
I
Is Internal Energy Always Constant in Thermodynamic Systems?
So, is the internal energy of a system always constant? I mean, if work done on the system is negative by convention. dU = dQ - pdV Then if work is done on the system, does that mean that it has to absorb heat to keep the internal energy constant? and if the system does work, it has to...- Inertigratus
- Thread
- Energy Internal Internal energy
- Replies: 5
- Forum: Classical Physics
-
P
How Are Internal Energy, Heat, and Work Related in Ideal Gases?
Can some one check my work, I'm not sure if I am understanding how internal energy heat and work are related. Work is defined to be positive if the system does work on the environment. Q=\Delta U +W I am showing if the process is positive, negative or 0. Also this is suppose to be an ideal gas- Punkyc7
- Thread
- Energy Heat Internal Internal energy Relationships Work
- Replies: 1
- Forum: Introductory Physics Homework Help
-
W
Factors affecting the internal energy
does the identity of molecules affect the internal energy, besides the temperature and volume?- weng cheong
- Thread
- Energy Factors Internal Internal energy
- Replies: 1
- Forum: Classical Physics
-
F
Does Total Internal Reflection Always Have Equal Angles?
If there is total internal reflection, does the angle of incidence always equal the angle of reflection? Thanks- fromthepast
- Thread
- Internal Reflection Total internal reflection
- Replies: 2
- Forum: Optics
-
F
Does Total Internal Reflection Occur for Light Passing from Air into Water?
Does total internal reflection occur if the refraction of light is passing from air into water? Thanks- fromthepast
- Thread
- Internal Reflection Total internal reflection
- Replies: 1
- Forum: Optics
-
G
Constant internal energy with volume change?
Constant internal energy with volume change?? My book says that you can change the temperature of a substance at constant internal energy by changing only the volume. how can this happen? since the internal Energy E is this: \Delta E = q + w = q - P\DeltaV where q is heat, w is...- gkangelexa
- Thread
- Change Constant Energy Internal Internal energy Volume Volume change
- Replies: 9
- Forum: Classical Physics
-
G
Phase Changes and internal energy
The internal Energy E is this: \Delta E = q + w = q - P\DeltaV where q is heat, w is work, and V is volume Phase changes occur when you change internal energy of the system, right? I am assuming that this means when you go from gas to liquid to solid, you must decrease the internal...- gkangelexa
- Thread
- Energy Internal Internal energy Phase
- Replies: 3
- Forum: Classical Physics
-
G
How Does the Magneto Caloric Effect Alter Internal Energy?
In magneto caloric effect, we see that when a substance in an adiabetic closure is exposed to external magnetic field, its temperature increases. But the internal energy of the substance has to be constant so this implies decrease in internal potential energy. However the application of magnetic...- gursimran
- Thread
- Change Energy Energy change Internal Internal energy
- Replies: 7
- Forum: Electromagnetism
-
L
Deriving an expression for internal energy.
Homework Statement Show that the change in internal energy of a simple system between states (V1, T1) and (V2, T2) is given by ∆U = \int^{T1}_{T2} C_v\ dT + \int^{V1}_{V2} T.\frac{\partial p}{\partial T}|_V - p \ dVHomework Equations dU=dQ-pdVThe Attempt at a Solution As U is a function of...- L-x
- Thread
- deriving Energy Expression Internal Internal energy
- Replies: 4
- Forum: Introductory Physics Homework Help
-
J
Setting up Fick's Law with Internal heating
I am solving for the flow of heat on a resistor, say it has a cross-section in the x-z plane and is extended along the y-axis. Fick's Law with internal heating is simply \frac{\partial T}{\partial t}=A\nabla^{2}T+BQ My PDE text gives Q as power delivered per unit volume. So substituting in...- jonthebaptist
- Thread
- Heating Internal Law
- Replies: 6
- Forum: Differential Equations
-
C
How do I know what the internal resistance of a battery is in a diagram?
Homework Statement I am trying to find terminal voltage of the batteries in the circuit shown. Homework Equations V = IR The Attempt at a Solution The solution manual says that it's the battery voltage, v, minus the voltage drawn from the resistance v = I * R. I understand this. I do not...- chiddler
- Thread
- Battery Diagram Internal Internal resistance Resistance
- Replies: 1
- Forum: Introductory Physics Homework Help
-

Conceptual question about internal energy
Hi there. Here is the deal, today morning in my thermodynamic class we were making some exercises of stability, and then surged a question about internal energy. The thing is I thought that the internal energy should be always positive in sign. This doesn't mean that the variation must be...- Telemachus
- Thread
- Conceptual Energy Internal Internal energy
- Replies: 2
- Forum: Calculus and Beyond Homework Help
-
S
False: Internal Energy of A & B is Not Determined by T Alone
Homework Statement Why is this false? "if systems A and B each consist of pure liquid water at 1 bar pressure and if TA>TB, then the internal energy of system A must be greater than that of B" Homework Equations The Attempt at a Solution It seems true to me. Could it be that B...- sparkle123
- Thread
- Energy Internal Internal energy
- Replies: 1
- Forum: Introductory Physics Homework Help
-

How to treat internal resistance in calculations ?
Homework Statement http://img217.imageshack.us/img217/654/circuit05.jpg Internal Resistance = 10 ohms (each) RL = 20 ohms R2 = 30 ohms R1 = 50 ohmsA) When the switch is open, calculate I B) When the switch is closed, calculate I Homework EquationsThe Attempt at a Solution First I'll try to...- Femme_physics
- Thread
- Calculations Internal Internal resistance Resistance
- Replies: 75
- Forum: Introductory Physics Homework Help
-
S
Understanding the Importance of Proper Lubrication for High Performance Engines
What are the uses of mineral oils and self contained lubrication system in internal combustion engine? Please answer fast Its urgent- sorayahya
- Thread
- Combustion Engine Internal
- Replies: 17
- Forum: Mechanical Engineering
-
U
Reaching any point mass configuration by internal forces
If we have N unique point masses, is it possible to use only internal forces (i.e. forces between each pair of masses) to reach any configuration of the points (modulo rotations and translations)? I assume this is well known, but don't know where to find a proof. Perhaps by induction from N=2...- uekstrom
- Thread
- Configuration Forces Internal Internal forces Mass Point
- Replies: 2
- Forum: Classical Physics
-
G
Internal Combustion Piston Lubrication
Hi, Can someone explain the lubrication mechanism for a piston in a typical automotive internal combustion engine? If the walls in the piston chamber are coated with oil, the lubricant should also burn along with the petroleum during the combustion phase (leaving problematic residues), so, is...- Grayfox
- Thread
- Combustion Internal Piston
- Replies: 7
- Forum: General Engineering
-
A
NON-monatomic gas expansion, find internal energy change
Homework Statement A NON-monatomic gas expands from I to F in the Figure (Since it is not monatomic U=3/2nRT does not give you the internal energy, this only works for monatomic gases, instead you must use the first law). The energy added to the gas by heat is 424 J when the gas goes from I to...- awertag
- Thread
- Change Energy Energy change Expansion Gas Gas expansion Internal Internal energy
- Replies: 1
- Forum: Introductory Physics Homework Help
-
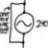
Total Internal Reflection Confusion
Homework Statement See figure attached. Homework Equations The Attempt at a Solution Initially, \theta_{c} = 60^{o} n_{glass}sin\theta_{c} = n_{air} So, n_{glass} = \frac{n_{air}}{sin\theta_{c}} Now I would think that the incident angle from glass to air would be...- jegues
- Thread
- Confusion Internal Reflection Total internal reflection
- Replies: 2
- Forum: Introductory Physics Homework Help
-
C
Thermodynamics - internal energy change question
Homework Statement Assume ideal gas conditions. Cp=(7/2)R, Cv=(5/2)R 15 moles of airis contained in a cylinder and prevented from escaping by a frictionless piston initial conditions are 1.5 bar, gas volume 0.2m^3 final conditions required are 10 bar t 27 degrees celsius calculate...- chriswilson
- Thread
- Change Energy Energy change Internal Internal energy Thermodynamics
- Replies: 2
- Forum: Introductory Physics Homework Help
-
C
Possible angles for internal gravity wave propagation
Homework Statement Internal gravity waves are generated along the coast of Norway by the principal lunar semidiurnal surface tide (known as the M2 tide) of period 12.42 h. If the stratification frequency N is 2 x 10-3rad s-1, at which possible angles can the energy propagate with respect to...- contempquant
- Thread
- Gravity Gravity waves Internal Waves
- Replies: 1
- Forum: Advanced Physics Homework Help
-
G
Internal E-field of dielectric cylinder immersed in static E-field
I am having trouble with a homework problem assigned by my physics professor. An infinitely long cylinder of radius a and relative dielectric constant Er is immersed in a static electric field E=Eox where x is supposed to denote a unit vector in the x direction. The cylinder is alligned on the...- grmitch
- Thread
- Cylinder Dielectric E-field Internal Static
- Replies: 6
- Forum: Advanced Physics Homework Help
-
K
Calculate emf and internal resistance with two circuits with the same cell
Homework Statement A cell is connected to a 10.0 Ohm resistor. The pd across the 10.0 Ohm resistor is 10.43 V. A 4.00 Ohm resistor is connected in parallel with the 10.0 Ohm resistor. The pd across the 10.0 Ohm resistor falls to 7.87 V. Calculate the emf and the internal resistance of the...- koalafacey
- Thread
- Cell Circuits Emf Internal Internal resistance Resistance
- Replies: 3
- Forum: Introductory Physics Homework Help
-

Does the Carnot heat engine law apply to an internal combustion engine?
The law says that the maximum thermal efficiency of a heat engine can be 88%. If I have and internal combustion engine using 9.8 cc/min instead of 25 to 30 cc/min and the other accepted figure is a 2L 4 cylinder engine for idle is it uses approx 5 HP of fuel just just to idle. So I am doing...- smokingwheels
- Thread
- Apply Carnot Combustion Engine Heat Heat engine Internal Law
- Replies: 21
- Forum: Thermodynamics
-

What is the Minimum Refractive Index for Total Internal Reflection?
Homework Statement See figure attached for problem statement. Homework Equations The Attempt at a Solution Using Snell's Law, n_{1}sin(\theta_{1}) = n_{2}sin(\theta_{2}) n_{1} = \frac{n_{2}sin(\theta_{2})}{sin(\theta_{1})} Where, \theta_{1} = 30^{o}, \theta_{2} =...- jegues
- Thread
- Internal Reflection Total internal reflection
- Replies: 5
- Forum: Precalculus Mathematics Homework Help
-
P
Change in internal energy when ice melts to water at 0C
If we melt ice at 0'C to water at 0C, what is the change in the internal energy of the ice-water system? As per the first law of thermodynamics, ΔU = ΔQ +ΔW where they are increase in internal energy, heat flow to the system and work done on the system respectively. If we melt ice at 0C, we...- Pranav Jha
- Thread
- Change Energy Ice Internal Internal energy Water
- Replies: 18
- Forum: Mechanics
-
S
Electric field in a conductor with an internal cavity
I'm not entirely sure if this is meant to be in the "advanced physics" section, it's for my undergraduate degree anyway. Homework Statement A conducting sphere of radius R, carries a total charge of q. It is surrounded by an electrically neutral conducting shell with an inner radius, a, and...- Silversonic
- Thread
- Cavity Conductor Electric Electric field Field Internal
- Replies: 2
- Forum: Advanced Physics Homework Help
-
R
Heat conduction through a slab with internal heat generation
Homework Statement There's a slab of a material with temperature T1 on the left and the T2 on the right. The thickness of the material is l with area A. In the centre, there is heat generation Qvol in the centre, which is a thin rod. Find the heat transfer Q through the material. Homework...- rafehi
- Thread
- Conduction Generation Heat Heat conduction Internal
- Replies: 5
- Forum: Advanced Physics Homework Help
-
F
What is the driving force to increasing the internal resistance of a battery
What is the "driving force" to increasing the internal resistance of a battery Homework Statement A brand new battery almost have no resistance, but overtime when the internal resistance is great enough, the battery "dies". So my question is, what is causing the internal resistance to...- flyingpig
- Thread
- Battery Force Increasing Internal Internal resistance Resistance
- Replies: 3
- Forum: Introductory Physics Homework Help
-
T
Help Internal Energy of a Liquid
In thermodynamics Change in Internal Energy = Heat - Work (\DeltaU=Q-W) During vaporization my Heat is mass*Latent Heat (Q=mL) and Work is Pressure*change in volume (W=P\DeltaV), so \DeltaU=mL-P\DeltaV. Let's take any of the natural monoatomic gases, Neon in this case. \Delta = Volume...- tamiller07
- Thread
- Energy Internal Internal energy Liquid
- Replies: 2
- Forum: Other Physics Topics
-
B
Find emf and internal resistance of a battery
Homework Statement When a 60 \Omega resistor and a 120 \Omega are connected in parallel across a battery there is a current of 700 mA flowing through the battery. When the 120 Ohms resistor is disconnected from the circuit the current through the battery drops to 500 mA. What is the emf and the...- Blu3eyes
- Thread
- Battery Emf Internal Internal resistance Resistance
- Replies: 8
- Forum: Introductory Physics Homework Help
-
U
Internal resistance, dual power source problem
Homework Statement An emergency supply consists of a small diesel engine driving an alternator. The output of the alternator is rectified and smoothed to produce DC. This has has to supply 10 amps of 110V emergency lighting and charge a 110V battery of internal resistance 2 ohms, If the...- ukdave
- Thread
- Dual Internal Internal resistance Power Resistance Source
- Replies: 2
- Forum: Introductory Physics Homework Help
-
B
Internal magnetic field experienced by H atom Electron
Homework Statement A 21 cm spectral line corresponds to the flipping of the electron in a hydrogen atom from having its spin parallel to the spin of the proton to having it anti-parallel. Find the internal magnetic field experienced by the electron in the hydrogen atom. Homework Equations...- Bosley
- Thread
- Atom Electron Field Internal Magnetic Magnetic field
- Replies: 1
- Forum: Advanced Physics Homework Help
-
S
Internal energy and thermodynamic entropy
As a practical matter, the internal energy of a system is treated as a state function of a the system and is concerned only with the kinetic energy of particles, not the potential energy. So for thermodynamic entropy, S=E/T, (S=entropy, T= absolute temperature) I'm considering E to be kinetic...- SW VandeCarr
- Thread
- Energy Entropy Internal Internal energy Thermodynamic
- Replies: 7
- Forum: Thermodynamics
-
K
Total internal reflection zero
How is that in refraction of light energy is lost but not in case of total internal reflection? Is the loss in total internal reflection exactly zero?