You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Melting Definition and 276 Threads
-
P
Melting Ice in Seawater: Cooling Steel on Seabed with 30kg Ice Block at -20degC
Using a 30kg cubic block Ice at -20degC, I want to cool down a piece of steel which is resting on the seabed. The mass of steel is 1kg and the ice block is place on top of the steel. The current sea temperature as well as the steel temp is estimated at 12degC Assuming that the piece of steel... -
J
Counter intuitive melting temperatures
I have heard that the melting/freezing point of helium is lower than that of hydrogen. Is this correct? if so why? My thoughts on this are that hydrogen has the lowest mass and therefore requires less energy than helium to be in a liquid or gaseous state, so it appears counter intuitive that...- JizzaDaMan
- Thread
- Replies: 5
- Forum: Thermodynamics
-
Q
Basic thermodynamic in chemistry, temperature of melting ice
Homework Statement Consider the heating curve for H2O. a. As heat is added to melt ice, does the the temperature change? Explain what happens to the heat that is added. b. For the same amount of H2O, does it take more to melt ice or vaporize water? Explain why. c. Is the fusion of ice...- qpham26
- Thread
- Replies: 4
- Forum: Biology and Chemistry Homework Help
-
S
Melting of ice with water thermodynamics problem
1. 0.500kg of ice at -5.00°C is put into a vessel containing 1.80kg of water at 17.00°C and mixed together, the result being a mixture of ice and water at 0.00°C. Calculate that final masses of ice and water, taking the water equivalent of the vessel as 0.148kg, the specific heat of ice as...- Satis24
- Thread
-
- Tags
- Ice Melting Thermodynamics Water
- Replies: 2
- Forum: Introductory Physics Homework Help
-
D
Solving Power Equation: Melting an Ice Cube
Power Equation?? Homework Statement An ice cube is placed in a microwave oven. Suppose the oven delivers 135 W of power to the ice cube and that it takes 33200 Joules to melt it. How long does it take for the ice cube to melt? Homework Equations P = W / T The Attempt at a Solution Ok so I... -
D
Predicting the Melting Point of Stereoisomers: A Scientific Inquiry
The melting point of N-acetyl-DL-alanine is 137◦Cand that of N-acetyl-D-alanine is 125◦C. What would you expect the melting point of N-acetyl-L-alanine to be or is this impossible to predict? Why? I thought that to approach this problem all that needed to be done is take the average of...- disneychannel
- Thread
-
- Tags
- Melting Melting point Point
- Replies: 8
- Forum: Chemistry
-

The Melting Point of Diamonds: Burning or No Burning?
I heard something that I adopted as a rule some years ago: "Every combustion produces H2O and CO2". I used it blindy until hydrogen burning came to mind. Clearly, H2 does not produce CO2. Now, what happens when a diamond reaches its melting point, and, does it burn afterwards? If so, what kind...- Weissritter
- Thread
-
- Tags
- Melting Melting point Point
- Replies: 8
- Forum: Chemistry
-
W
Temp & Melting Point of Water: Pressure Effects Explained
assuming water at atmospheric conditions, now if the pressure is reduced, at some lower point of pressure, water starts to boil.at this point, will the temp of water change and why?- wave525
- Thread
-
- Tags
- Melting Melting point Point
- Replies: 12
- Forum: Other Physics Topics
-
P
Total entropy change for ice melting in a room
Homework Statement Okay, so I am having difficulties with understanding the concepts around entropy, take this question: What is the total entropy change for 7 kg of ice melting from -5 C° to 5 C° in room at 5 C°. Homework Equations dS=dQ/T Q =mΔH m*c*ln(tfinal/tinitial) c_ice=2 c_water=4...- plpg
- Thread
-
- Tags
- Change Entropy Ice Ice melting Melting
- Replies: 4
- Forum: Introductory Physics Homework Help
-
V
Are superconductivity and melting not understood?
Hi all, I've been told from a couple different people something along the lines of "we don't fully understand how melting (the phase transition) works". Same with superconductivity. But I thought melting was fairly straightforward: The molecules in the material keep gaining energy until...- VortexLattice
- Thread
-
- Tags
- Melting Superconductivity
- Replies: 5
- Forum: Atomic and Condensed Matter
-
J
Reference Request: Understanding Metal Melting
Apologies in advance for the abstract nature of this question; I don't know enough about this field to be more detailed. I'm trying to understand the behavior of metal when heat is applied to it. I'd like to be able to answer such questions as: how fast does the metal melt? what is the effect on... -
H
MHB Calculating the Rate of Decrease in Diameter of a Melting Snowball
Volume of a sphere: \frac{4}{3}\pi r^{3} Volume of a sphere in terms of diameter: \frac{\pi d^{3}}{6} Rate of volume: \frac{d}{d(time)}volume = -1 cm^{3}/min. Rate of diameter: \frac{d}{d(time)}10 cm = ? I'm not sure what to do at this point -
R
Reduce Melting Temp. by applying Pressure
Homework Statement The molar volume of ice at 273.15K and 101.33 kPa is V1=19.6 cm3 That of water V2=18.00 cm3. Latent heat of melting of ice is L=6.0 kJ mol-1. Find the pressure that must be applied to reduce the melting point by 1 K. Homework Equations Clausius-Clapeyron...- Ryomega
- Thread
- Replies: 5
- Forum: Advanced Physics Homework Help
-
J
IM forces-substances of increasing melting points
Q.In which of the answers below are the substances listed in order of increasing melting point? a) Cl2 < CHF3 < H2O < CHCl3 < SiO2 b) Cl2 < CHCl3 < CHF3 < H2O < SiO2 c) Cl2 < CHF3 < CHCl3 < H2O < SiO2 d) Cl2 < H2O < CHF3 < CHCl3 < SiO2 e) SiO2 < H2O < CHCl3 < CHF3 < Cl2 How can we tell...- joe98
- Thread
-
- Tags
- Increasing Melting Points
- Replies: 1
- Forum: Chemistry
-

Is it Possible to Make an MWIR Lens from Melted Table Salt?
I was thinking of making an MWIR lens from table salt, which is quite transparent for it. That needs a big, monolithic cylinder of salt - 3x1 cm. Now, growing it the classic-oversaturated-solution way would take forever, if feasible at all. And i want to try to just melt some salt and cast... -
P
What Is the Difference Between Triple Point and Melting Point?
Hello, Im new here and I hope someone of you can answer this probably trivial question. I tried to find the answer in many of phyisc/termodynamic texbooks but in vain. http://imageshack.us/photo/my-images/62/123002h.jpg/ http://imageshack.us/photo/my-images/62/123002h.jpg/ According to...- Paul Snasel
- Thread
- Replies: 2
- Forum: Thermodynamics
-
S
Effects of soluble impurities boiling and melting points
In my textbook, it says that impurities lower the melting point and increase the boiling point. But is this only true if the boiling point of the impurity is greater than the boiling point of the solvent, and the melting point is greater than of the solvent too? So essentially, if greater... -
E
Effect of pressure on Melting point
Hey, I have read that INCREASING the external pressure on solids INCREASES their melting point.(except for ice) WHY DOES THIS HAPPEN? (This is similar to the effect on Boiling point which rises when external pressure is increased. I know this happens because boiling takes place at temp...- emailanmol
- Thread
- Replies: 9
- Forum: Mechanics
-
S
Ice cube melting, solving for Initial Temp of Ice.
Homework Statement .019 Kg icecube @ unknown temperature (In deg C) Placed in 200 mL (.2 KG) water at 35 C. After melting, water is at 22.4 C. Find initial temperature of ice using conservation of energy. Homework Equations I've done the math a ton of times and keep getting -60 C... -
S
Thermal stability vs melting point
An ionic compound is more thermally stable when the metal cation is more reactive. So its harder to decompose. But then what's the difference between melting and decomposition? Also, when an compound is harder to decompose, does it mean its melting point is higher? Because even though Na2O... -
S
How to tell if ionic compound has a higher melting point?
I know that MgO has a higher melting point than NaCl as Mg is Mg2+ while O is O2- while Na is Na+ and Cl ia Cl-. but in CuCO3 and Na2CO3, Na2CO3 has a higher melting point compared to CuCO3. So I'm not sure how to tell which ionic compound will have a higher melting point than another when they... -
S
Boiling and melting point of impure substances
They say that an impure substance has an increased boiling point and reduced melting point (i know that they will melt/boil at a range of temperature). But does impurity mean that its melting and boiling point is higher than the substance itself or it doesn't matter? I think that the impurity... -
C
Transition metals melting points
I found this on a webpage: In any transition element series, the number of unpaired electrons first increases from 1 to 5 and then decreases back to the zero .The maximum five unpaired electrons occur at Cr WHY?' doesn't it occur at MN?? -
P
Is there a type of plastic that has a low melting point or low soft temperature?
Is there a type of plastic that has a low melting point or better a low soft temperature? What we need is to blow up a ball (3-7 cm in diameter), cover the ball except a 6mm hole, let it dry, and then removes the plastic ball through the 6mm hole. So to remove the plastic it needs to be soft...- peterje
- Thread
- Replies: 9
- Forum: Materials and Chemical Engineering
-
F
Melting Ice with Tape: Exploring Chemical/Electric Solutions
Lets say I have some tape with powder copper inside the glue of the tape (doesn't have to be copper). The goal is to heat the tape so that its capable of melting small amounts of ice on the surface of the tape. The tape is wrapped around a surface about 3" tall 1/4" thick and 8" wide. On the...- FireScript
- Thread
- Replies: 18
- Forum: General Engineering
-
I
Why is there a difference ?Melting Point of Lead & Tungsten: Find RMSA
Homework Statement Hi all, I have a question which is regarding melting point. I know that lindemann's criterion doesn't actually describe the phenomena of melting but is an approximation of what maybe occurring/ an intuitive approach. So the question is in a tabulated form for lead and...- ibysaiyan
- Thread
-
- Tags
- Melting Melting point Point
- Replies: 1
- Forum: Advanced Physics Homework Help
-
B
Glass, melting points, and Xmas gift idea
Hey, I recently bought my gf a ring and would like to put it in a glass bottle and allow it to hang by a fish line with it corked on top. I haven't found any wine bottles that allow the ring to just go in naturally, I have been having to heat all of them. I was wondering if I were to... -
L
1 kg of melting ice and 1 kg of boiling water are mixed
1. 1 kg of melting ice and 1 kg of boiling water are mixed: Which temperature will have the mixture when all ice is liquefied ? I don't know if there is formula The attempt solution: I don't know this question is really hard there are not enough information -
P
Correlation between metal strength and melting point?
I am unsure of this matter and so I am curious, do stronger metals more than likely have higher melting/heating points? Does a metal that takes more force/pressure to break or snap have a higher melting/heating point as well? Do metals/alloys like steel or titanium have higher melting points...- Physics quest
- Thread
- Replies: 5
- Forum: Other Physics Topics
-
G
How Does a Steel Bar Sink Through Ice?
Homework Statement Question no. 4 in this document (there's a helpful picture, too): Homework Equations The Clausius-Clapeyron equation: \frac{\delta p}{\delta \tau}=\frac{l}{\tau ∆v}, where v is the volume per unit mass, i.e., the inverse of the density. The Attempt at a... -
G
Melting Points: Solids in Tube - Will Temp Remain Same?
I have 2 solids in a tube. If i start heating the recipient, will the temperature remain the same at the melting point temperature of the substance with the highest melting point? -
G
Melting point of the naphthalene [something went wrong]
hello, Last day in school we were heating solid naphthalene until it melted, but the temperature didn't stop at the melting point. if anybody has any idea of what could happen, please tell me, maybe we didn't do it correctly, or the test tube was dirty or whatever... Thanks- germangb
- Thread
-
- Tags
- Melting Melting point Point
- Replies: 2
- Forum: Chemistry
-
C
How Do You Calculate Entropy Change When Melting Ice at Varying Temperatures?
Homework Statement 1. Homework Statement Calculate the entropy change when 1 mole of ice at 268 K is melted to form water at 323 K. The heat capacity of ice is 3.8 J K-1 kg-1 and that of water is 75 J K-1 kg-1. The enthalpy of fusion of ice at 273 K is 6.02 kJ mol-1. I know the entropy...- chriswilson
- Thread
- Replies: 1
- Forum: Introductory Physics Homework Help
-
C
Calculation of entropy change for melting ice
Homework Statement Calculate the entropy change when 1 mole of ice at 268 K is melted to form water at 323 K. The heat capacity of ice is 3.8 J K-1 kg-1 and that of water is 75 J K-1 kg-1. The enthalpy of fusion of ice at 273 K is 6.02 kJ mol-1. I know the entropy change by the melting of...- chriswilson
- Thread
-
- Tags
- Calculation Change Entropy Ice Melting
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
R
OpAmp LM-324 Melting Due to ESD?
Hi gents. In a rig several times an OpAmp LM-324 burns and melts (and support too ! ). The circuit works perfectly for many hours and suddently ... I must precise that high voltage ( -6 kV) is not very far. My question : is ESD able not only to destroy OpAmp but also to make it melting ?- Ravaner
- Thread
- Replies: 5
- Forum: Electrical Engineering
-
C
1.5 cubic meters ice cube melting
Hi, I'm new on this forum. We have some science days in our school, and there is a big 1.5x1.5x1.5m ice cube melting. There is a contest who will guess the aprox. time that it takes to melt. I think it will be more than two days, but I would like to get a more concrete answer somehow... :)... -

Impurities Impact Melting & Freezing: Explained
"For a pure substance, melting occurs at the same temperature as freezing." Does it means that if impurities are added, then the melting point of the substance is not at the same temperature as freezing ? If yes, how you would explain this ?- Jadaav
- Thread
-
- Tags
- Freezing Impact Impurities Melting
- Replies: 7
- Forum: Thermodynamics
-
C
Metals question - melting bismuth onto aluminum
hey guys, i am planning on buying some pieces of bismuth, as well as machining a disc of aluminum so that there is a hollow circular 'canal' which goes concentric on the disc aluminum has a melting point of 660.32 degrees celcius, for bismuth it is 271.5 degrees celcius i also have a very... -
Y
Impurities on melting point and boiling point of water
When impurities was added to the water, it tends to increase the boiling point of the water to 102 degree celcius and lower the melting point of the water to -2 degree celcius! Why this happen? Is it because the impurities tends to absorb the heat supplied to boil the water causing it to take in... -
A
Higher melting point, PH3 or NH3?
Hi everyone, I have a question for all of you In an exercise of chemistry it's written to find the compound, between every couple, which has the highest melting point. The three couples are: H20 H2S; KBr CF4, NH3 PH3. In the first case water has higher melting point because the bond is...- ANDR3W
- Thread
-
- Tags
- Melting Melting point Point
- Replies: 4
- Forum: Chemistry
-
C
Melting point of lead at 100atm
Homework Statement Homework Equations use clausius-clapeyron equation: \frac{dp}{dt} = \frac{L}{T(V_{2}-V_{1})} which can be rearranged (i think) in the following: p = p_{0}+\frac{L}{\Delta V}ln(\frac{T}{T_{0}}) The Attempt at a Solution so using the above equation i solved...- curto
- Thread
-
- Tags
- Lead Melting Melting point Point
- Replies: 14
- Forum: Introductory Physics Homework Help
-
E
Effect of pressure on melting point.
Is there a quantifiable value for a given substance that correlates to the degree by which pressure has an effect on the element or substance melting/boiling point? Allow me to elaborate. If the substance in question is known (silicon dioxide, for example), how could we calculate the... -
M
Quiver path algebra and F-term relations in melting crystals
EDIT: fixed TeX issues Hi, I'm learning about the correspondence in string theory between the geometry of Calabi-Yau manifolds and melting crystals. I care more about the math and know almost nothing about string theory, so navigating the literature littered with so much string theory jargon... -
E
Melting a dangerous asteroid about to impact Earth
I was wondering how much energy it would take to melt a big enough asteroid considered to be dangerous? Is it even possible? If possible, could it be done fast enough before it impacts? If it is completely melted can it then be somehow dispersed so that it turns into smaller chunks that... -
S
Melting Point: How Heat Changes Liquids to Solids
Name a substance that will change from liquid state to solid state on heating.- sam013024
- Thread
- Replies: 10
- Forum: Other Physics Topics
-
P
What is so magical about the melting and boiling points?
Melting and boiling explanation based on the kinetic-molecular model have always confused me. I am in grade 12 and still don't feel that I have got any real understanding of the process. Something that has always seemed odd to me is that the idea of increase in potential energy is only mentioned...- Pranav Jha
- Thread
- Replies: 17
- Forum: Other Physics Topics
-
C
Exploring the Melting of an Ice Cube in Water
Homework Statement an ice cube is melted in water which is continuously stirred to be at a constant temperature of 0 degrees. the stirring is gentle enough so the work done is negligible. my question is why in this case does the heat come from the air to melt the ice cube and not the...- Chronos000
- Thread
- Replies: 1
- Forum: Advanced Physics Homework Help
-
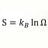
Heat Transfer Coefficient for Ice Melting Time
Homework Statement One liter of water at 30 C ( 30000 calories ) 100 gram (100 cc ) sphere of ice at 0 C is in center of water volume. The ice will absorb 8000 cal melting and final water temp = 22000 cal/ 1100 g = 20 C Assuming mixing and uniform water temp during melting ,and vessel is...- morrobay
- Thread
- Replies: 1
- Forum: Advanced Physics Homework Help
-
G
How to determine which molecule has the highest melting point?
The question is: Which substance has the highest melting point? (A) CO (B) CO2 (C) SiO2 (D) P2O5 The answer is C but I don't know why (you obviously can't look up the values). I need to be able to figure these types of questions out regardless of the...- gsingh2011
- Thread
- Replies: 1
- Forum: Chemistry
-
A
Solving for Melting Ice: 500g Lead & -10C Ice Block
Homework Statement a 500 g lead mass is heated to 150 C and placed on a block of ice at -10 C. how much ice, if any will melt? 52 kg = block of ice Homework Equations The Attempt at a Solution i didint know where to start so i did qmcdeltaT + qmcdeltaT = 0, and i failed at it i...