You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Moles Definition and 207 Threads
Moles are small mammals adapted to a subterranean lifestyle (i.e., fossorial). They have cylindrical bodies, velvety fur, very small, inconspicuous ears and eyes, reduced hindlimbs, and short, powerful forelimbs with large paws adapted for digging.
The term mole is especially and most properly used for "true moles" of the family Talpidae in the order Eulipotyphla, which are found in most parts of North America, Europe and Asia, although it may also refer to unrelated mammals of Australia and southern Africa that have convergently evolved the "mole" body plan.
Moles are known pests to human activities such as agriculture, lawncare, and gardening. They do not eat plant roots, but cause damage indirectly by eating earthworms and other small invertebrates in the soil. While moles may be viewed as pests, they do provide many positive contributions to the soil, gardens, and ecosystem, including soil aeration, feeding on slugs and other small creatures that do eat plant roots, and providing prey for other wildlife.
View More On Wikipedia.org
The term mole is especially and most properly used for "true moles" of the family Talpidae in the order Eulipotyphla, which are found in most parts of North America, Europe and Asia, although it may also refer to unrelated mammals of Australia and southern Africa that have convergently evolved the "mole" body plan.
Moles are known pests to human activities such as agriculture, lawncare, and gardening. They do not eat plant roots, but cause damage indirectly by eating earthworms and other small invertebrates in the soil. While moles may be viewed as pests, they do provide many positive contributions to the soil, gardens, and ecosystem, including soil aeration, feeding on slugs and other small creatures that do eat plant roots, and providing prey for other wildlife.
View More On Wikipedia.org
-
S
Chemistry Question regarding Chemistry ( Moles
Homework Statement A silicon chip used in an integrated circuit of a computer has a mass of 4.86 mg. How many silicon atoms are present in this chip? Homework Equations no. of moles of element = no. of particles / 6.22 * 10^23 particles No. of moles of a element : mass of element...- SiriusA
- Thread
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
N
Avogadro's constant, number of moles, number of particles
Dealing with these has reminded me why I hated chemistry. What is the relationship between the above 3? Given atomic weight of nitrogen and Avogadro's constant, can the number of moles be found? -
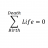
Chemistry Calculating Precipitate and Moles: Na2S + Co(NO3)2
Homework Statement ##300.0 mL## of ##1.50 \times 10^{-10} M## ##Na_2S_{(aq)}## is combined with ##200.0 mL## of ##1.50 \times 10^{-10} M## ##{Co(NO_3)_2}_{(aq)}##. Determine what precipitates. Determine how many moles precipitate. Homework Equations The Attempt at a Solution...- STEMucator
- Thread
-
- Tags
- Moles
- Replies: 7
- Forum: Biology and Chemistry Homework Help
-
D
Chemistry Amount of moles emitted by burning oil
Homework Statement Calculate the number of moles of CO2 that are emitted into the atmosphere per year due to the total energy consumption on Earth (16TW). Note: 1kg oil yields 42MJ and for oil C:H ' 1:2. I don't know how to calculate how many grams/moles of CO2 are emitted by burning 1kg of...- Donna14
- Thread
- Replies: 4
- Forum: Biology and Chemistry Homework Help
-

Number of moles equation in exhaust gas
I've been given information that the exhaust gas contains only water and no HydroCarbons. So,the only hydrogen composition found in the exhaust gas is in the water. Similarly,the hydrogen in the air coming-in is only found in the water molecules as the incoming air is pure and without any fuel...- marellasunny
- Thread
- Replies: 3
- Forum: Mechanical Engineering
-
C
[Thermal] Is it possible that increases of moles decrease entropy?
From Statsitical And Thermal Physics (Reif. international edition 1985) 160 page. (5.4.4) S(T,V;√) = √[∫{cv(T`)/T`}dT` + Rln(V) - Rln(√) + constant] (integral is from T0 to T , cv is specific heat) This is a entropy of system for temperature 'T' , Volume 'V' , Moles '√' <--... -
O
Chemistry Calculate Moles of H2 from 6 Moles of H20
Homework Statement Suppose you have 6 moles of H20. You want to calculate how many moles of H2 you have. how many is it? Homework Equations Avagradro's number: 6.02*10^{23} The Attempt at a Solution I got to the equation: 2H_2+O_2\rightarrow 2H_2O I kind of figured out...- oneplusone
- Thread
-
- Tags
- Moles
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
F
Chemistry Calculating Moles of Fuel Burned with Volume Concentration
Homework Statement "1.79 g of a fuel was burned in the presence of oxygen. The fuel is a composite of 90% octane and 10% ethanol. Calculate the number of moles of this fuel that was burned."Homework Equations Moles = Mass/Molar Mass The Attempt at a Solution 90% of 1.79 g is 1.61 g. This was...- FredericChopin
- Thread
-
- Tags
- Calculation Chemistry Moles
- Replies: 4
- Forum: Biology and Chemistry Homework Help
-
B
Calculating Moles Ratio of Reactants for Yield f in K Equilibrium
If I want to calculate the number of moles ratio of reactants which, in a reversible reaction of equilibrium constant K, will give me a certain yield 'f', what is the mathematical definition of the yield in this case? For instance, in the reaction A + B ⇔ C + D, let's say we can denote extent... -

Chemistry Can I Convert Moles Between Products?
Homework Statement Hi! I was assigned a problem for chemistry like this: 2 A + B → 2 C + 3 D It asks to find mol of C if you start with 0.320 mol D.. Can I convert between C and D? I know that I can convert between A and B.. but I'm not sure about C and D. Homework Equations...- rakeru
- Thread
- Replies: 2
- Forum: Biology and Chemistry Homework Help
-
K
Understanding Moles From Ideal Gas Law
Hi All, I would like to understand a little more about the following equation moles = \frac{Mass(g)}{Molar Mass} I know that this is to calculate the Moles for use in the ideal gas law equation PV=n \times R \times T Lets say I had to find two variable in the ideal gas law...- Kayne
- Thread
- Replies: 7
- Forum: Other Physics Topics
-
C
Chemistry Mg, N2, O6 Moles: How Many Do You Have?
1. If I have this chemical formula : Mg(NO3)2 and I have one mole of it, how many moles of each element do I have ? Mg: 0,16mol N2: 0,19 mol O6: 0,65 mol Homework Statement Would my answer be any good ?- chemistry1
- Thread
-
- Tags
- Moles
- Replies: 7
- Forum: Biology and Chemistry Homework Help
-
M
Chemistry How many moles of MnO4- were added?
Hello, I have no idea how to solve this... The initial mass of an unknown sample containing iron (II) is 0.512 g. 12.6 mL of 0.01522 M KMnO4 is required to titrate the unknown to the endpoint. a) How many moles of MnO4- were added? b) How many moles of iron (II) must be present in the...- MrPoison
- Thread
-
- Tags
- Moles
- Replies: 4
- Forum: Biology and Chemistry Homework Help
-
Y
Chemistry Why Is ln(N) Around 50 for Various Chemical Compositions?
Homework Statement Estimate the number N of molecules in a few grams of matter. Explain why in that case ln (N) is of the order of 50 whatever the chemical composition. Homework Equations M = m/N where M is the molecular mass The Attempt at a Solution I'm really unsure about the entire...- Yuriick
- Thread
-
- Tags
- Moles
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
A
Chemistry How Do You Calculate Iron Concentration in Tablets from AAS Data?
A group of students decided to use AAS to analyze three different brands of iron tablets to determine their content. They placed a tablet in a 100 mL beaker with 6 mol/L HCl and covered it with a watch glass and heated slowly for 15 min. They then filtered the solution into a 250 mL volumetric...- ASidd
- Thread
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
D
Chemistry Equivalence point concentration/number of moles
Homework Statement Fe[3+]+V[2+]-->Fe[2+]+V[3+] If you start with 0.1000 M solutions and the first-named species is the titrant, what will be the concentration of each reactant and product at the equivalence point of the titrations above? Assume that there is no change in [H+] during the...- drcasey
- Thread
-
- Tags
- Equivalence Moles Point
- Replies: 4
- Forum: Biology and Chemistry Homework Help
-
3
Solving 2.0 Moles of Monatomic Gas & Solid Thermal Interaction
Homework Statement 2.0 moles of a monatomic gas interacts thermally with 2.0 moles of an elemental solid. The gas pressure decreases by 50 degrees C at constant volume. What is the temperature change in the solid? I missed this day in class and I have no idea where to even begin...- 39643
- Thread
- Replies: 2
- Forum: Introductory Physics Homework Help
-

Chemistry What is the number of moles of OH- in 0.3 L OF 0.005 M Ba(OH)- ?
Homework Statement *0.3 L OF 0.005 M Ba(OH)- What is the number of moles of OH- in 0.3 L OF 0.005 M Ba(OH)- ?2. The attempt at a solution In 1 mol ba(oh)2--------> 2 mol OH- so In 0.005 M ba(oh)2 ----------------> 2 x 0.005 M OH- I know I am doing something wrong. please help thanks in...- AakashPandita
- Thread
-
- Tags
- Moles
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
O
Chemistry Calculate Reactant Concentration from Chemical Equilibrium Moles
If someone could check my work on this, I'd appreciate it: Thanks!- ohms law
- Thread
- Replies: 8
- Forum: Biology and Chemistry Homework Help
-
A
Chemistry How to Find Initial Moles of Reactants for Given Reaction | Moles Homework
Homework Statement We are given a reaction: H2O2 + 2I- + 2H+ → 2H2O + I2 and are asked to find the initial number of moles of each reactant. V=10mL and C=0.2 mol/L for potassium iodide in the solution. V=5mL and C=0.5 mol/L for sulfuric acid in the solution. V=5mL and C=0.02 mol/L for...- anthonych414
- Thread
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
S
Chemistry Calulating Atomic Percentages given moles and weights of compounds
Homework Statement This is the statement out of a paper: Slurry 10 grams of ZnS with 3mL of .2N Cu(CH3COO)2 solution (corresponding to 0.6 atom % Cu) and 1.7 mL of .2N NH4Cl solution (corresponding to 0.34 atom % Cl) So my question is how did they calculate the .6 at. % and .34 at. %...- Squall
- Thread
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
X
Chemistry How many moles of valence electrons are in 1 mole of nitride ions?
Homework Statement The number of moles of valence electrons in 1 mole nitride ions are? Homework Equations Nitride ion- N3- The Attempt at a Solution The number of electrons are 8. Therefore number of moles should also be 8?- xiphoid
- Thread
- Replies: 5
- Forum: Biology and Chemistry Homework Help
-
E
Calculating the Mass of 100 Trillion Uranium 238 Atoms | Physics Homework
Homework Statement What would be the mass of one hundred million million (100 000 000 000 000 = 1014) atoms of Uranium 238 (atomic mass is 238)? Homework Equations The Attempt at a Solution I tried finding the mass of each particle by diving the avogadro constant by 238.. The...- ehabmozart
- Thread
- Replies: 1
- Forum: Introductory Physics Homework Help
-
S
Find moles of MNo4; % uncertainty and % error
My group messed this experiment up and now i have to get a good grade(i receive 50% on all of my labs!). I would highly appreciate your input. Our teacher gave us a procedure sheet, which we "accurate followed" This is what we did:(If this is not enough i have a few more steps that were done...- SinOfLiberty
- Thread
-
- Tags
- Error Moles Uncertainty
- Replies: 1
- Forum: Chemistry
-
G
Solving Moles Confusion: Question about Hydrocarbon Burning
Hi guys, I have been solving many stoichiometry problems but their is something I can do but I don't fully understand why it works it kinda confuses me atleast on mathematical level. I don't like doing something I don't fully understand. What I don't understand is if we have for example a...- Genericcoder
- Thread
-
- Tags
- Moles
- Replies: 7
- Forum: Chemistry
-
A
Avogadro's Principle and number of moles + thermodynamics problem
Hi, I learned recently Avogadro's principle which states " At constant temperature and pressure, equal volumes will contain the same number of molecules" this can be translated to if you double the volume, you double the number of moles. Now i don't get that at all! where does the extra...- Awesome-o
- Thread
-
- Tags
- Moles Principle Thermodynamics
- Replies: 1
- Forum: Introductory Physics Homework Help
-
S
Converting the molarities into moles but I'm having trouble with the reaction
Can anyone help me with this problem? Find the pH when 45.0 mL of 0.250 M NaOH(aq) is added to 35.0 mL of 0.200 M HCl(aq). I started by converting the molarities into moles but I'm having trouble with what the reaction should look like. Shouldn't it produce a salt (NaCl) and water? And... -
H
Chemistry Calculate Moles of NaOH: Homework Help
Homework Statement Calculate the moles of NaOH in the reaction of NaOH + acetic acid. This is a titration where the NaOH neutralized the acetic acid. It took 0.02 L of NaOH to neutralize 3 ml of acetic acid. I just do not know what to do, have been trying for a few days and can't seem to...- homevolend
- Thread
-
- Tags
- Moles
- Replies: 4
- Forum: Biology and Chemistry Homework Help
-
G
Chemistry Partial pressure and moles problem?
Homework Statement Here is the problem: Carbon monoxide and oxygen are kept at exactly 300 K in separate chambers of the apparatus shown below. When the stopcock is opened, the combustion reaction begins and is allowed to proceed to completion, while being maintained at exactly 300 K...- gh_pluvilias
- Thread
- Replies: 2
- Forum: Biology and Chemistry Homework Help
-
P
Determine Moles of Gas in a Container
Homework Statement 10. An ideal gas in a container has a pressure of 2.50 atm and a volume of 1 m3 at a temperature of 30oC. How many moles of gas are in the container? a. 20 moles b. 45 moles c. 62 moles d. 83 moles e. 100 moles Homework Equations PV=nRT The Attempt at a...- physgrl
- Thread
- Replies: 1
- Forum: Introductory Physics Homework Help
-
J
Chemistry Calculating Moles Using Volume and Molarity
Homework Statement Was wondering if I did this right, don't know if I used the right equation. Equation: Ba(NO3)2 + Na2(SO4) = Ba(SO4) + 2Na(NO3) Volume of barium nitrate: 5mL Molarity of Barium nitrate: .1M Homework Equations Molarity = moles/L The Attempt at a Solution...- jubbly
- Thread
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
S
Chemistry Find number of moles of HCl to change pH of Histidine buffer.
Homework Statement There is 400 mL of 0.2 M histidine buffer- pH 11. Find the moles of HCl that are needed to change the pH to 3.2. Homework Equations pH= pKa+log[b]/[a] The Attempt at a Solution First, this is how you would go from acid to base (acid-->base): 1. There is 400 mL of 0.2 M... -
C
Chemistry Understanding Moles: A Homework Challenge
Homework Statement i have just learned that one mole is actually what i had learned as one molecule. now one atom is also known as one mole.eg. one mole of methane has 5 moles of atoms( this sentence is written by my teacher and no matter how i try to look at it from different perspectives...- Celluhh
- Thread
- Replies: 5
- Forum: Biology and Chemistry Homework Help
-
D
Chemistry Relationship between moles of O2 and Ch2 Units in a combustion reaction
Homework Statement I want to find a relationship between the number of moles of O2 used and the number of CH2 units in an alkane in a complete combustion reaction. Homework Equations The Attempt at a Solution Right now I have come up with a math equation, given X units of CH2...- deezer
- Thread
- Replies: 4
- Forum: Biology and Chemistry Homework Help
-
N
Calculating Moles and Molecules of Oxygen in a Gas Cylinder
Homework Statement A gas cylinder contains of 0.8 × 10^-3 m^3 volume oxygen.the temperature of the oxygen is 320k,and the pressure of the gas is 1.5 × 10^6 Pa.how to calculate: number of moles and molecules of Oxygen? mass of Oxygen if its molar mass is 32.0 × 10^-3 kg ? The mass of a single...- nafo man
- Thread
- Replies: 10
- Forum: Advanced Physics Homework Help
-
C
Chemistry How Many Moles of Oxygen Gas Are Produced from Decomposing Hydrogen Peroxide?
Homework Statement Calculate the number of moles of oxygen gas produced from the completely catalyzed decomposition of 6.60ml sample of a 3.5% solution of H2O2. The density of the 3.5% solution of H2O2 is 1.01 g/ml. Homework Equations 2(H2O2) --> 2(H2O) + O2 p=m/v The Attempt...- chakakhan
- Thread
- Replies: 4
- Forum: Biology and Chemistry Homework Help
-
E
Chemistry Nomenclature, molecular mass, moles help (attempted problems already)
Alright, below are screen captures of some work that I'm tackling. They show my attempts and answers at the problems. I would like to know whether I'm correct or wrong and how to correct the mistakes. The one I need most help with is, the one where you're given density and asked to find...- Edin_Dzeko
- Thread
-
- Tags
- Mass Molecular Moles Nomenclature
- Replies: 4
- Forum: Biology and Chemistry Homework Help
-
L
How Can I Determine the Volume of a Diatomic Ideal Gas in a Rigid Container?
A diatomic ideal gas is in a rigid container at an initial sate of 300K and 1.3x10^5 Pa is heated to a final state of 330K by 623.25J of heat. calculate the pressure in the container after the heating, the work done by the gas, the change in internal energy and the volume of the container. if...- Latios1314
- Thread
- Replies: 5
- Forum: Introductory Physics Homework Help
-
N
Chemistry How Do You Calculate Moles from Grams for BaCl2 *2H2O?
If I have BaCl2 *2H2O how does that come to 8.46507394x10-3 Here is the question: A common laboratory experiment involves the analysis of a mixture of BaCl2 and BaCl2 *2H2O. you are given a white powder that is a mixture of these compounds and are asked to determine the weight percent of...- nieuport27
- Thread
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-

Chemistry Pb(NO3)2 0.16M: Molar Concentration of Ions
Homework Statement What molar concentration of each ion appear in 0.16M Pb(NO3)2? *Note* The title incorrectly says "moles". Homework Equations M = mol/L The Attempt at a Solution My teacher tells me this is wrong, but I can't see my fault: Molar Mass of Pb+2 = 207.20g Molar Mass of NO3-...- AJKing
- Thread
-
- Tags
- Moles
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
S
Chemistry Which Method for Calculating Moles is More Effective?
Do both of these methods for calculating the number of moles work? we've learned a new method recently which i prefer over the older method for current curriculum n1=c1v1 n for number of moles, c for concentration 1 and v for volume 1 and the old method is # of moles = mass/molar...- supernova1203
- Thread
-
- Tags
- Moles
- Replies: 4
- Forum: Biology and Chemistry Homework Help
-
N
Chemistry Calculating Moles of Fe in 400.0g of Iron
Homework Statement How many Fe atoms and how many moles of Fe atoms are in 400.0 g of iron? The Attempt at a Solution Ok. i attempted to find the number of moles by using 400/55.8 to get 7.17 but that is wrong?! please tell me ehy this is wrong. i set up a proportion like this...- name_ask17
- Thread
- Replies: 6
- Forum: Biology and Chemistry Homework Help
-
T
10 moles of an ideal gas has a gauge pressure of 2 atm what is new T? no clue
10 moles of an ideal gas has a gauge pressure of 2 atm what is new T?? no clue [b]1. Homework Statement [/b 10 moles of an ideal gas has a gauge pressure of 2 atm and a temperature of 200 K. If the volume of the gas is doubled and the pressure dropped to 1 atm., what is the new temperature...- teggenspiller
- Thread
- Replies: 14
- Forum: Introductory Physics Homework Help
-
P
Chemistry Calculating Moles in C1V1=C2V2 Reactions
Just a quick question, when calculating C1V1 = C2V2, do I need to account for Mole Rato? Say that C1 had 1 moles required in reaction as opposed to C2 which required 2 moles, would I just multiply C2 by 1/2?- Procrastinate
- Thread
- Replies: 5
- Forum: Biology and Chemistry Homework Help
-

Chemistry Calculate Volume of Products in NH3 + Cl2 Reaction
Homework Statement A mixture is completed from 15.0L of ammonia and 15.0L chlorine measured at the same conditions, these compounds react according to the following equation 2NH3(g) + 3Cl2(g) yields N2(g) + 6HCl(g) When the reaction is completed, what is the volume of each gas? Assume...- Duderonimous
- Thread
- Replies: 9
- Forum: Biology and Chemistry Homework Help
-
Q
Chemistry Calc Mass A -> Moles B: 2.5x10^5 kg H2SO4->?Moles H3PO4
Equation: 3H2SO4 + Ca3(PO4)2 + 6H20 -> 3[CaSO4×2H2O] + 2H3PO4 Problem: If 2.5×10^5 kg of H2SO4 react, how many moles of H3PO4 can be made? Equations used: 1) kilograms to grams (multiply kilograms by 10^3) 2) Mass to moles (divide given mass by mass of one mole) 3) Moles of substance A to...- Qube
- Thread
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
A
Pressure in cylinder, find moles of air entered. Please help
Pressure in cylinder, find moles of air entered. Please help! :) Homework Statement The inside of the cylindrical can shown above has cross-sectional area 0.0044 m2 and length 0.28 m. The can is filled with an ideal gas and covered with a loose cap. The gas is heated to 385 K and some is... -
I
Chemistry Calculating Moles of O2 Gas at Pressure & Volume
Homework Statement what is the number of moles of o2 gas at a pressure of 760 cm of hg in a container of volume 24.63 l at 27 degree c? i read its answer... now i want to ask is this question theoratical or can be calculated ?- intellpuneet
- Thread
- Replies: 4
- Forum: Biology and Chemistry Homework Help
-
P
Weird: number of moles of NaOH in 20 ml of 1M NaOH
Hi there, this is a not a homework question but rather question about the theory behind this problem. Say if someone asks you to calculate the number of moles in 20 ml of a 1M NaOH solution, I would usually say it is going to be 20 mmol as you do 20 ml/1000 ml/L x 1M = 0.02 moles = 20 mmol... -
E
Chemistry How Do You Calculate Moles in a CaCO3 and HCl Reaction?
Hi I'm stuck on a homework question on moles. I know the mole formula, but the question doesn't state any needed numbers. Question: Calculate the number of moles of calcium carbonate and hydrochloric acid at the start of the reaction. the reactions is CaCO3+2HCL---CaCl2+CO2+H2O How...- Elizabeth12
- Thread
-
- Tags
- Moles
- Replies: 2
- Forum: Biology and Chemistry Homework Help