You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Phase change Definition and 142 Threads
In chemistry, thermodynamics, and many other related fields, phase transitions (or phase changes) are the physical processes of transition between the basic states of matter: solid, liquid, and gas, as well as plasma in rare cases.
A phase of a thermodynamic system and the states of matter have uniform physical properties. During a phase transition of a given medium, certain properties of the medium change, often discontinuously, as a result of the change of external conditions, such as temperature, pressure, or others. For example, a liquid may become gas upon heating to the boiling point, resulting in an abrupt change in volume. The measurement of the external conditions at which the transformation occurs is termed the phase transition. Phase transitions commonly occur in nature and are used today in many technologies.
View More On Wikipedia.org
A phase of a thermodynamic system and the states of matter have uniform physical properties. During a phase transition of a given medium, certain properties of the medium change, often discontinuously, as a result of the change of external conditions, such as temperature, pressure, or others. For example, a liquid may become gas upon heating to the boiling point, resulting in an abrupt change in volume. The measurement of the external conditions at which the transformation occurs is termed the phase transition. Phase transitions commonly occur in nature and are used today in many technologies.
View More On Wikipedia.org
-

Question on entropy in adiabatic phase change
Homework Statement Consider a closed, adiabatic system consisting of a mixture of liquid and solid substance Z at equilibrium at its melting point. Z (solid) <---------> Z (liquid) Which of the following statements is true regarding the system? A) The entropy of the system is at a maximum...- RoboNerd
- Thread
- Replies: 8
- Forum: Biology and Chemistry Homework Help
-

Converting torque to electricity
We have a solar tracking device that uses phase change of paraffin wax when heated inside a solar receiver. With a helical slot in a torque tube we can translate the expansion into rotational motion. We use this rotation to point solar panels at the sun, thus generating more electricity. Look...- Jeffb47
- Thread
- Replies: 7
- Forum: Mechanical Engineering
-
N
Thermodynamics phase change question
My thermo teacher was talking about 2 phase rankine cycles and he said the once water boils at atmospheric pressure its temperature can not get higher than 212 no matter how much heat you add. He said the only way you can raise the temperature of steam past 212 F is the raise the pressure. Why...- nate9519
- Thread
- Replies: 2
- Forum: Mechanical Engineering
-
F
Release or absortion of energy on bonds and phase change
I believe I can explain why there is energy needed to break intermoleculares bonds and getting into a gas or liquid, but the other way around confuses me. Bonds have potential energy associated to it, so It's needed work to break the bonds, because we would be trying to move a molecule away from... -

Thermodynamics relating to temperature and phase changes V.2
Homework Statement Suppose a room with 75 m3 of air also contains 80 kg of glycerol and the initial temperature in the morning is 16 °C. If 1.2 kWh of heat is added to the room between morning and afternoon, calculate the final temperature of the air in the room in the afternoon. Use 18 °C for...- Mnemonic
- Thread
- Replies: 1
- Forum: Introductory Physics Homework Help
-
E
Discovering a Unique PCM: High Volume Change, Low Heat Storage Capacity
I'm trying to find a Phase Change Material (PCM), but the entire industry is pretty one-minded when it comes to what it should be used for - energy storage. You can see by the wording of the Wikipedia article: https://en.wikipedia.org/wiki/Phase-change_material that they list advantages like...- eknifff
- Thread
-
- Tags
- Pcm Phase change
- Replies: 5
- Forum: Materials and Chemical Engineering
-
Z
Can a Steel Vessel Contain Liquid Hydrogen at 15,000 PSI and 25C?
I've read a fews post about liquid hydrogen boil off, but could use a bit more clarification; insight on the following thought experiment would be really helpful: What happens if I take a 1 L steel vessel, put 1 L of liquid H2 into it, seal it off, and let it sit in a room at 25C? The density... -
B
Are Alternative Kinetic and Hydro Storage Solutions Viable for Solar Energy?
I have seen a lot of effort go into finding cheap, safe, high energy density phase change materials to store energy in the form of heat so power produced during the daytime may be delivered at night time. But I have an issue with storing energy in phase change materials. They rely on chemicals...- BernieM
- Thread
- Replies: 14
- Forum: General Engineering
-

What Is the Boiling Temperature of the Liquid Using Van der Waals Equation?
Homework Statement The bulb of a constant volume gas thermometer is immersed in an ice/water/water vapour mixture at equilibrium and the recorded pressure is 0.400 atm. It is then immersed in a boiling liquid and the pressure is 0.844 atm. Sufficient gas is then removed from the bulb such that...- VoteSaxon
- Thread
- Replies: 2
- Forum: Advanced Physics Homework Help
-

Pressure, Temperature and Fluid phase change
Some please clear my confusion here, when you compress a fluid the temperature increases. Since the temperature increases (fluid gets warmer), why is the fluid condensing from gas to fluid? when temperature increases, isn't that the fluid will evaporate? This has been bugging for awhile...- Nicky_Boy02
- Thread
- Replies: 4
- Forum: Mechanical Engineering
-
S
Mix steam with colder water, what is final temp?
Homework Statement 100 g of steam at 100C is mixed with 1 kg of water at 10C. What is the final temperature of the mixture? (in C) heat capacity of water: cw = 4.186 kJ kg-1 K−1 latent heat of vapourisation for water: Lv = 2256 kJ kg−1 latent heat of fusion for water: Lf = 334 kJ kg−1 Homework...- SunshineCat
- Thread
- Replies: 2
- Forum: Introductory Physics Homework Help
-
A
How Does Adiabatic Expansion Lead to Condensation?
Homework Statement In an earlier part of the question, I derived the temperature dependence of the latent heat of vapourisation of a liquid as dL/dT=L/T+ΔCp-L/Vvap(∂Vvap/∂T)p I am asked to find the condition that upon expanding the gas adiabatically, we get condensation to occur, by considering...- albega
- Thread
-
- Tags
- Change Expansion Phase Phase change
- Replies: 9
- Forum: Introductory Physics Homework Help
-
F
Velocity vs time graph simple harmonic motion phase constant
Homework Statement http://i.imgur.com/u8vUv5a.jpg Find the phase constant. Homework Equations x(t)=Acos(ωt + Φ) v(t)=-Aωsin(ωt + Φ) Vmax = ωA ω=2π/T The Attempt at a Solution ω = 2π/12 = 0.5236 A = 60/0.5236 = 114.59 cm v(0) = -30 = -114.59(0.5236)sinΦ 0.5 = sinΦ Φ = π/6 and 5π/6. Which...- firezap
- Thread
- Replies: 6
- Forum: Introductory Physics Homework Help
-
T
Latent Heat in Solid-->Liquid transitions (phase change)
Homework Statement Show that in Solid to Liquid transitions at T \ll {T}_{c} , L\simeq constant where {T}_{c}, L are the critic temperature and latent heat respectively. Homework Equations \left( \frac{d ( \frac {L} {T})} {dT} \right) = \frac {{c}_{p2}-{c}_{p1}} {T}+ \frac...- thonwer
- Thread
- Replies: 1
- Forum: Introductory Physics Homework Help
-
D
Find the temperature change of a person struck by lightning
Homework Statement A lightning flash releases about 1010J of electrical energy. If all this energy is added to 50 kg of water (the amount of water in a 165-lb person) at 37∘C, what are the final state and temperature of the water? The specific heat of water is 4180 J/kg⋅∘C, heat of...- dashortkid
- Thread
- Replies: 1
- Forum: Introductory Physics Homework Help
-
P
Latent Heat Problem? Not sure how to solve
Homework Statement A window of a car is found to be covered with 0.8cm of ice. If the area of the window is 1 m2, and the ice is at -12 degrees Celsius, what is the minimum heat required to melt all the ice? Take the density of ice to be 900 kg/m3, the specific heat capacity of ice to be 0.5...- PBryan833
- Thread
-
- Tags
- Heat Latent heat Phase change
- Replies: 4
- Forum: Introductory Physics Homework Help
-
T
Calorimetry with Phase Change: Predicting Final Phase and Temperature
Homework Statement An aluminum cup (of negligible mass) is filled with 0.5 kg of water with a temperature of 15◦C and 1.3 kg of ice (at -8 celsius) is added. A)What is the final phase or phases of the mixture? B)What is the final temperature of the mixture? C)How much heat is required to raise...- toothpaste666
- Thread
- Replies: 14
- Forum: Introductory Physics Homework Help
-
H
Confusion regarding Standing Waves and Reflection resulting in Phase Change
So a standing wave is one which looks to be standing still at certain harmonic frequencies. At these frequencies, when the wave reflects off the end, it will undergo a phase change of 180 degrees. This creates constructive interference as shown below: In the case of a wave on a string, since...- Hereformore
- Thread
- Replies: 2
- Forum: Optics
-
R
Questions Regarding Effect of Nucleation in Phase Change
1. Is nucleation a phenomenon that occurs in all phase change (freezing/melting, evaporation/condensation)? 2. I've always read evaporation/condensation described as a liquid-vapour interface phenomenon (water molecules going entering-leaving the interface at equal rates in equilibrium). If...- Red_CCF
- Thread
- Replies: 2
- Forum: Classical Physics
-
M
Light's Refractive Index & Phase Change: Explained
Consider a plane beam of light is falling normally on a material with thickness (d) and refractive index (n), the light partially transmits and partially reflects the light, so the light transmitting the light travels a distance (2nd) coming out of the material have a phase change of ∏ always...- manimaran1605
- Thread
- Replies: 1
- Forum: Other Physics Topics
-
S
Calculation of volume after phase change
Folks, It's been a while since I've done calculations like this so I was wondering if someone can help me calculate the volume of a gas at a specific temperature as compared to its liquid volume when measured below its boiling point. I can't seem to find a phase diagram for this material, is...- spiri
- Thread
- Replies: 1
- Forum: Materials and Chemical Engineering
-
J
Effect of phase change in wave function on energy
I understand that a local gauge transformation functions to conserve the energy of an electron as it moves through space/time. What I don’t understand is why the energy of the electron, as dictated by the momentum and potential energy terms of the Schrödinger equation changes as a function of...- jordankonisky
- Thread
- Replies: 1
- Forum: Quantum Physics
-
N
Electric field Phase change upon rarer to denser reflection of an EM
Can anyone please provide an intuitive explanation of why phase shift of 180 degrees occurs in the Electric Field of a EM wave,when reflected from an optically denser medium and also why the phase remains same on reflection from a denser to rarer medium? please try to explain on an atomic/sub...- ns_phonon
- Thread
- Replies: 1
- Forum: Electromagnetism
-
T
Ionization driven phase change
Greetings, I've been searching online without success for information related to the process (name?) Of utilizing the repusive force between negatively ionized particles to facilitate a change of phase from liquid to plasma. I would like to be able to take a substance in liquid form inside a...- taylaron
- Thread
- Replies: 1
- Forum: Other Physics Topics
-
C
Including a heat for a phase change or not?
Hi PF! I just took a test for my class and there is one problem I can't get my mind off of. I'm not necessarily looking for the correct solution here, just if my thinking was correct. It went more or less like this: there is 40g of ice at 0 degrees C in 200g of water in an aluminum calorimeter...- catzmeow
- Thread
-
- Tags
- Change Heat Phase Phase change
- Replies: 6
- Forum: Introductory Physics Homework Help
-
M
Phase change in lock-in amplifier
I just started to use a lock-in amplifier, and I am trying to following the procedures by adjusting zero phase. But my input phase changes with time and sensitivity, and I couldn't lock in onto it. The question is, is this normal that phase changes all the time during the experiment or if there...- Mikech
- Thread
- Replies: 1
- Forum: Electrical Engineering
-
T
Finding Ionizable Phase transition agent with low phase change energy
Hello everyone, I'm designing an experiment which utilizes a single agent in different phases (liquid, gas, and plasma). The apparatus needs to be able to ionize the gas into a plasma (with +/ - 1 or more electrons). I'm trying to keep energy consumption at a minimum, so current phase... -
S
What does metal gas look like microscopically?
I was thinking of a substance I could use to describe particle arrangement in solid/liquid/gas phases to school kids, and after realising water would be bad to use (since the liquid is denser than solid), I thought of using metal elements since they can exist in all phases. Basically the... -
D
Amount of CO2 vaporized in a phase change from liquid to solid & vapor
Homework Statement Saturated liquid CO2 is at T= 293 K and P=5.72*10^6 Pa and undergoes throttling to P=1.01*10^5. The resulting solid and vapor mixture is at T = 195 K. What fraction of the carbon dioxide is vaporized? (The enthalpy of the saturated liquid in the initial state is 24,200...- doombanana
- Thread
- Replies: 1
- Forum: Advanced Physics Homework Help
-
R
Kinetic energy change during phase change
hi all, its been hours that i could not find a decent answer for a 'simple' Q: during a phase change (say, boiling) the temp' doesn't change, as we all know. we also know that the temp' is a measure of the system kinetic energy (KE). im interested to know how the average KE AND its... -
J
Sub Nitrogen Phase Change Compressor unit
Hey guys, I don't post here often but I like to come here when I have a problem that really rattles my brain. I am an 'overclocker' by hobby (I tune computers), a big part of the overclocking scene is cooling the processor of the computer as far as possible. For years now the 'best' way of...- jebusv20
- Thread
- Replies: 1
- Forum: General Engineering
-
A
Heat and phase change: latent heat
Homework Statement A 42kg block of ice at 0°C is sliding on a horizontal surface. the initial speed of the ice is 7.3 m/s and the final speed is 3.5m/s. Assume that the part of the block that melts has a very small mass and that all the heat generated by kinetic friction goes into the block...- alaa410
- Thread
- Replies: 4
- Forum: Introductory Physics Homework Help
-
K
Solving Phase Change & Spatial Separation with Wavelength & Velocity
Homework Statement A wave of wavelength 75 cm has velocity 375 m/s. a. What is the spatial separation between two points that are 30° out of phase at a particular time? b. What is the phase change at a particular position for a time change of 0.5 ms? Homework Equations u = λ / T...- kgal
- Thread
- Replies: 1
- Forum: Introductory Physics Homework Help
-
X
Work done on steam during phase change?
Homework Statement (a) How much work is done on the steam when 3.89 mol of water at 100°C boils and becomes 3.89 mol of steam at 100°C at 1.00 atm pressure? (Assume the latent heat of vaporization of water is 2.26 106 J/kg.) (b) Assume the steam to behave as an ideal gas. Determine the...- XianForce
- Thread
- Replies: 4
- Forum: Introductory Physics Homework Help
-
A
Is the Phase Change Floatation Cycle a Theoretical Flaw in Thermodynamics?
Consider the following theoretical cycle. There should be a flaw somewhere, but I could not find it yet and will appreciate it if someone can point it out to me. A lot of ideal assumptions are made, such as reversible heat transfer, but it is not intended to be practical, as long as it adheres...- Ambforc
- Thread
-
- Tags
- Change Cycle Phase Phase change
- Replies: 8
- Forum: Classical Physics
-
J
Phase change of reflected wave - is there a polarization dependence?
Does a light wave in air (n1 = 1) that is reflected off a glass surface (n2=1.5) experience a 180deg phase change? Looking at the Fresnel equations (theta = 0deg) I learn that: according to http://en.wikipedia.org/wiki/Fresnel_equations rp = (n2-n1)/(n1+n2) = 0.2 and rs = (n1-n2)/(n1+n2)... -
K
Phase change from mass in middle of a string
Homework Statement There is a mass M in the middle of an infinite string, of the same linear density on both sides. The reflection coefficient is r=-ip/(1+ip) where p=ω^2 M/2Tk How does the phase change on reflection vary with M, for fixed ω and T? Homework Equations Phase change...- Koranzite
- Thread
-
- Tags
- Change Mass Phase Phase change String
- Replies: 1
- Forum: Advanced Physics Homework Help
-
D
Phase Change and Lowest Boiling Point
I am studying for the MCAT and I am having a hard time understanding the rationale for the following question from my review: Q: One of the limitations of using a gas as a shock absorbing cushion is that under high pressure, the gas may liquefy and lose compressibility. Which of the... -
A
Guage symmetry - invariance under arbitrary phase change
As I understand it the heart of gauge symmetry is that I can change the phase at different points different amounts and the Lagrangian/action is unchanged. What I am not clear on is whether the changes I can make are completely arbitrary - I can make any change I want at any point - or whether...- arlesterc
- Thread
- Replies: 6
- Forum: Quantum Physics
-
M
Why is there a phase change in reflection and why is it always \pi?
Hi, When there is reflection, we generally use the phase shift upon reflection to be \pi. Where does this \pi come from or is it arbitrary? I ask because I came across an optics book which describes beam splitters (a mirror is of course a beam splitter with reflectivity, R=1 and...- McLaren Rulez
- Thread
- Replies: 6
- Forum: Optics
-
S
Explain phase change of an EMW in terms of photons.
Hi Guys, I have a question. Can we attempt to explain the phase change of a carrier wave in terms of photons? For example consider GPS signal. It is BPSK modulated, in which phase change of 180 degrees happens for multiple of 1540 cycles of the carrier. How is the phase information...- sriecewit
- Thread
- Replies: 2
- Forum: Quantum Physics
-
M
Understanding Phase Change: Effects of Heat on Kinetic Energy
When a substance undergoes a phase change, the added heat changes (a) the temperature, (b) the kinetic energy, (c) the potential energy, (d) the mass of the substance. I believe the answer is kinetic energy because the molecules move faster.- mopar969
- Thread
-
- Tags
- Change Phase Phase change
- Replies: 5
- Forum: Introductory Physics Homework Help
-
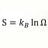
Heat Transfer Coefficient for Phase Change
Homework Statement One liter of water at 30 C (30000 calories ) A 100 gram sphere of ice at 0 C in center of water volume . The ice will absorb 80000 calories melting, and final water temperature = 22000 cal/1100g = 20 C. Assume mixing and uniform water temp during melting and water vessel...- morrobay
- Thread
- Replies: 1
- Forum: Engineering and Comp Sci Homework Help
-
C
Phase change resulting in pressure
Homework Statement The density of water at 0 C is 999.8 kg/m^3 while that of ice at 0 C is 917.0 kg/m^3. What is the pressure generated by expanding ice as it freezes in a biological cell whose major component is water? Assume the cell to be a sphere of radius 5 micrometres. I have started...- chris_0101
- Thread
-
- Tags
- Change Phase Phase change Pressure
- Replies: 1
- Forum: Introductory Physics Homework Help
-
S
Phase Change over Distance: Understanding k and l in Wave Analysis
kl gives the phase change over a distance l. I need an explanation. I understand that k is the wavenumber. In other words, it is a measure of the number of cycles of a wave (in terms of radians) per unit length. Therefore, kl gives the number of cycles traversed over a distance l. How...- spaghetti3451
- Thread
-
- Tags
- Change Phase Phase change
- Replies: 1
- Forum: Other Physics Topics
-
L
Phase Change Problems: How to Solve for Heat Release and Temperature Changes
Homework Statement I have three questions I need help on. Can someone please explain to me how to do them, how to correctly set up the problem, and what I need to know. I have a test tomorrow. Thanks! Q1: How much heat is released freezing 75 grams of steam at 110 degrees C into ice at...- lolphysics3
- Thread
-
- Tags
- Change Phase Phase change
- Replies: 1
- Forum: Introductory Physics Homework Help
-
N
Thermodynamics, melting, phase change
Homework Statement If ice is at(-12) deg c and has a mass of .55593125kg, how much heat is required to melt the ice. Homework Equations Q=mcT The Attempt at a Solution i tried Q=mc(water)T and Q=mc(ice)T but both were marked wrong. can you show me what I am doing wrong here?- name_ask17
- Thread
- Replies: 2
- Forum: Introductory Physics Homework Help
-
S
How is Heat Transfer Calculated in Ice Melting Problems?
1. An amount of ice of mass 80.0g is in a freezer at a temp of -15 degree Celsius. The ice is removed from the freezer, and a total of 19.2KJ of heat is added to the ice. Determine the final temperature of the ice and the mass of ice (if any) which remains after the heat is supplied. 2...- sdevgon
- Thread
- Replies: 4
- Forum: Introductory Physics Homework Help
-

Phase change in heat engines & heat pumps
This question has been in the back of my mind for a while. In short, is having a phase change between liquid and vapor more desirable for a heat engine or for a heat pump? Clearly, having a large area enclosed by the loop in a P-V diagram increased the amount of work in the cycle, so...- Redbelly98
- Thread
- Replies: 4
- Forum: Mechanical Engineering
-
M
Latent heat and phase change question
Homework Statement A 100g cube of ice at 0 degrees is dropped into 1.0kg of water that was originally at 80 degrees. What is the final temperature of the water after the ice has melted? Homework Equations Q = ml Q = mc(Tf - Ti) The Attempt at a Solution i'm not sure again about...- mizzy
- Thread
- Replies: 5
- Forum: Introductory Physics Homework Help