Reactions Definition and 625 Threads
-
S
Reversible and Irreversible reactions
There are 2 types of reactions 1) Being fully irreversible and 2) reversible So for 1) the graph of Gibbs energy against the progress of reaction would look like this: http://postimg.org/image/phycri0vr/ So the difference between 100% product and 100% reactant is the ΔG of the reaction...- sgstudent
- Thread
- Irreversible Reactions Reversible
- Replies: 6
- Forum: Classical Physics
-
W
What Factors Affect the Speed of Oxidation/Reduction Reactions?
In Ox/Red reactions there 3 factors that can speed up a reaction between 2 compounds: the first being a greater concentration, which is pretty easy to comprehend. What I don't understand is how heat affects the speed of a reaction ( at a micro level) and how is adding a third component to...- WavesOfPhysics
- Thread
- Factors Reactions Speed
- Replies: 7
- Forum: Chemistry
-
E
Is the concept of binding energy in nuclear reactions contradictory?
Hello forum. So the other day I was pondering properties of atomic nucleus, in particular the property of binding energy (mass defect). Whenever a nucleus -- through a nuclear reaction of some sort -- is split or joined into a more stable nucleus, energy is released. However the newly formed...- eroxore
- Thread
- Energy Nuclear Reactions
- Replies: 7
- Forum: High Energy, Nuclear, Particle Physics
-
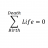
Nuclear Reactions: Why Deuterium Can't Undergo Beta Decay
Homework Statement Explain why deuterium cannot undergo ##\beta## decay or produce a stable nucleus while tritium can. Homework Equations Deuterium : ##{^2_1}{H}## Tritium : ##{^3_1}{H}## The Attempt at a Solution Observe the negative ##\beta## decay of deuterium : $${^2_1}{H}...- STEMucator
- Thread
- Nuclear Reactions
- Replies: 6
- Forum: Introductory Physics Homework Help
-
C
Addition reactions: Alkenes vs Alkynes
Homework Statement Alkenes are more reactive than alkynes toward addition of electrophilic reagents like HCl. Yet when alkynes are treated with one molar equivalent of these reagents, it is easy to stop the reaction at the alkene stage. This appears to be paradox. Explain. The Attempt at a...- consciousness
- Thread
- Addition Reactions
- Replies: 6
- Forum: Biology and Chemistry Homework Help
-
L
Standard reduction potential and half reactions
Homework Statement Determine what redox reaction, if any, occurs (at 25° C) when lead metal (Pb) is added to 1.0-M solution of NiCl2 Homework Equations Ecell = Ecathode- Eanode Here is standard reduction potentials given for Pb and Cl Pb2+(aq) + 2e- → Pb(s) E°(V) = -0.13 Cl2(g) 2e-...- leroyjenkens
- Thread
- Potential Reactions Reduction Standard
- Replies: 2
- Forum: Biology and Chemistry Homework Help
-
L
The electrical energy produced from chemical reactions
Hi there, new member here (**TL,DR is available at the bottom of this post**) As you may or may not know, if you were to put in a plate of zinc at one end and a plate of copper at the other, put them in an acid solution, electricity will be produced. More of that stuff here...- longchair
- Thread
- Chemical Chemical reactions Electrical Electrical energy Energy produced Reactions
- Replies: 3
- Forum: Electromagnetism
-

Chemical Kintetics - Sequential reactions
Chemical Kinetics - Sequential reactions Homework Statement For a reaction ##A \rightarrow B \rightarrow C##, ##t_{1/2}## for A and B are 4 and 2 minutes respectively. How much time would be required for the B to reach maximum concentration. Homework Equations The Attempt at a...- Saitama
- Thread
- Chemical Reactions
- Replies: 5
- Forum: Biology and Chemistry Homework Help
-
S
Precipitation reactions with AgNO3 and NaCl
When we add them together, AgCl precipitates as AgCl is insoluble in water so their interactions would cause them to form a solid. However, isn't the interaction between Na+ and Cl- greater than in Ag+ and Cl-? As NaCl has a stronger lattice energy than AgCl. So why is AgCl insoluble while NaCl... -
A
Direction of normal reactions on a beam
Homework Statement Problem 10.36 The bar has cross-sectional areaAand modulus of elasticity E. If an axial force F directed toward the right is applied atC, what is the normal stress in the part of the bar to the left ofC? (Strategy: Drawthe free-body diagram of the entire bar and write the...- arestes
- Thread
- Beam Direction Normal Reactions
- Replies: 1
- Forum: Introductory Physics Homework Help
-

Differential equations for series variable volume reactions
How do you make a differential equation for such? Say for example. I have two reactions in series, A → R and R → S going in a gas-phase reaction. If I'm correct, the ODE for the conversion of A is dXA/dt = kA*[(NAo/vo)*(1-XA)/(1+δA*YAo*XA)]*[(vo/NAo)*(1+δA*YAo*XA)]. I don't know now...- maistral
- Thread
- Differential Differential equations Reactions Series Variable Volume
- Replies: 13
- Forum: Materials and Chemical Engineering
-
S
Nucleophilicity in substitution reactions
Is there any absolute order of the nucleophilicity of nucleophiles participating in organic substitution reactions or is it dependent on solvent,substrate or any other factors ? If so,how? -
G
Predict Reactions & Macromolecules for Benedict's, Iodine & Bauret Tests
So I am asked this question in biology lab we aren't provided by any information to how to solve it,but we are asked to research on the internet in order to solve it. Predict the reactions you would expect to see for the following foods if they were tested using benedict's test, iodine (I2KI)...- Genericcoder
- Thread
- Reactions
- Replies: 1
- Forum: Biology and Medical
-
M
Another: Simple Displacement Reactions & Balancing Chemical Reactions
Homework Statement Complete the simple displacement reactions and balance any chemical reactions. Homework Equations Pb + NaCl → __________ + ____________ The Attempt at a Solution Pb + NaCl → PbCl + Na But I don't know which charge of lead to use: Pb2+ or Pb4+ to...- mdsa12
- Thread
- Chemical Chemical reactions Displacement Reactions
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
M
Simple Displacement Reactions & Balancing Chemical Reactions
Homework Statement Complete the simple displacement reactions and balance any chemical reactions. Homework Equations copper + silver nitrate → __________ + ____________ The Attempt at a Solution Since copper on the periodic table can be Cu2+ or Cu+, my solution was copper + silver...- mdsa12
- Thread
- Chemical Chemical reactions Displacement Reactions
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
R
Reactants of CO2: NaOH & H2CO3 |Chemical Reactions
I want to ask that which element can react with CO2 gas to split c and o or what will happen if NaOH reacts with h2co3 NaOH+H2CO3---> 2CH3COONa+2H2O+5O2 IS IT CORRECT?- rajavarshney
- Thread
- Co2 Reactions
- Replies: 2
- Forum: Biology and Chemistry Homework Help
-
A
Looking for a better understanding of chemical reactions
This is for self study. 1. Why don't some molecules react? For example, NH3 + CaCO3 2. Where can I find a good list of standart state enthalpy and entropy values for molecules? My Chem I teacher gave us all a pretty good handout, but that's been a while ago, and I don't know where it is... -
J
How to Classify Reactions: Hydrolysis, Ionization, and Self-Protolysis
Homework Statement Hello, I'm trying to learn how to classify reactions. I want to know how do I tell ionization, self protolysis and hydrolysis. Homework Equations NH4(aq) + H20(l) <--> NH3(aq) + H30(aq)+ This is hydrolysis why? How does it differ from this hydrolysis...- Jbreezy
- Thread
- Classification Reactions
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
J
Predict the product of the following reactions:
Homework Statement Predict the major organic product of the following reactions: The Attempt at a Solution For 1, I got: And for 2, I got: If anyone can help confirm, I'd be much obliged! Our professor is crazy and gives these types of problems for practice...- Jormungandr
- Thread
- Product Reactions
- Replies: 2
- Forum: Biology and Chemistry Homework Help
-
K
How to find support reactions in frames
how to find support reactions in frames- karimullah
- Thread
- Frames Reactions Support Support reactions
- Replies: 2
- Forum: Mechanics
-
M
Nuclear Reactions, Can anyone check my solution?
Homework Statement A nuclear power station reactor using 235 U (Uranium) as fuel has an output of 107 W. How much uranium is consumed per hour if the overall efficiency is 10%. The 235 U decays by the following reaction: n + 235 U → 144 Nd + 89 Y + (3)(n) + (7)(e-)Homework Equations P = E /...- maceng7
- Thread
- Nuclear Reactions
- Replies: 1
- Forum: Introductory Physics Homework Help
-
I
Doubts about potential energy, bonding and chemical reactions
Hello everybody, First, sorry for the bad english, it is not my language. I have a few theoretical problems and I would be very thankful if you helped me. 1º: I see the graphic about covalent bonds and potential energy a lot of times and they always say that potential energy... -
S
Photoatomic and photonuclear reactions
Grettings to all, I'm interested to know which are the main interaction between: 1.gamma-atom, also known like photoatomic reactions 2.gamma-nuclei, photonuclear reactions. e.g photofission Thank you!- Stephan_doc
- Thread
- Reactions
- Replies: 4
- Forum: Nuclear Engineering
-
T
Sn1 Reactions: Substrate structure vs leaving group stabilit
Homework Statement Let's say I have two compounds in an identical solvent. The compounds are also identical except for the following: One has a Br bonded to a tertiary carbon, and the other has an I bonded to a secondary carbon. Which would react first in an Sn1 nucleophilic reaction...- ThousandFjord
- Thread
- Group Reactions Structure Substrate
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
S
Calculating support reactions in a beam
Homework Statement Trying to calculate the support reactions here. Homework Equations ƩFy = 0 ƩMA = 0The Attempt at a Solution Well I've come up with the first equation (I assume two are needed to solve for both support reactions from the above equations). ƩFy = 0 RA + RB (up) = 10kN +...- Studious_stud
- Thread
- Beam Reactions Support Support reactions
- Replies: 7
- Forum: Engineering and Comp Sci Homework Help
-
B
Kinetics, Complex Reactions, Approximations
I wasn't sure where to ask this question as I don't know where else to make requests like this, so I thought I would go here. Please feel free to move it to a more appropriate forum if there is one. My syllabus states the following point: ------ Analysis of complex reactions using...- Big-Daddy
- Thread
- Complex Kinetics Reactions
- Replies: 2
- Forum: Biology and Chemistry Homework Help
-
M
Find units of rate constants and write reactions
Homework Statement Find the units of k1, k2, k3, k4, and k5. Write rate reactions for dA/dt a. A\leftrightarrowB k1 (forward) k2 (backward) b. A + B \rightarrow C k3 (forward) c. A + R \rightarrow 2R k4 (forward) d. A \rightarrow B k5 (forward) Homework Equations...- marimari
- Thread
- Constants Rate Reactions Units
- Replies: 11
- Forum: Biology and Chemistry Homework Help
-
M
Calculating energy released during fusion reactions
Homework Statement A Sun-like star may convert some carbon 12C (nuclear mass 1.998 ×10-26 kg) to oxygen 16O (nuclear mass 2.657 ×10-26 kg) towards the end of its life. Approximately how much energy is released per fusion reaction? Homework Equations e=mc2 c+he = o + energy The...- Meganwright
- Thread
- Energy Energy released Fusion Reactions
- Replies: 2
- Forum: Advanced Physics Homework Help
-
P
Find the reactions the pin A and roller D exert on the truss
Homework Statement The rigid truss supports the loads shown. Find the reactions the pin A and roller D exert on the truss. All concentrated loads act on the pins joining the truss membersHomework Equations ƩMa= -5ft(3kips)-10ft(2kips)-.707(1kip)10ft+Dy(15ft) Dy=2.80 kips*ft The Attempt at a...- pb23me
- Thread
- Pin Reactions Roller Truss
- Replies: 2
- Forum: Introductory Physics Homework Help
-
L
Spontaneous Reactions and Enthelpy
Today in AP Chemistry we started learning about entropy and spontaneous reactions. Could someone explain on an undergraduate level (preferably not using calculus) how, if the universe has a fixed amount of mass and energy, that reactions can be spontaneous and create their own energy? Thanks! -
D
Gaseous Chemical Reactions and Volumes
(Hi everyone! Apologising for the trivial(and likely boring) question in advance. Sadly, it has me boggled for some reason). Homework Statement In a certain temperature and under a pressure, 500 mL of H2, and 100 mL of O2 are poured into a container. A Chemical reaction occurs as follows...- danielakkerma
- Thread
- Chemical Chemical reactions Reactions Volumes
- Replies: 4
- Forum: Biology and Chemistry Homework Help
-
H
Electrode Potentials and Redox reactions
What is the relation between Electrode Potentials and Redox reactions ? Can we determine the relative strength of reduction of a metal by looking just at the electrode potentials ? Here is a particular thing that confuses me : We know that the standard electrode potential of Lithium is... -
C
Trying to understand causes and effects of nuclear reactions and radioactivity
I have been reading a lot about nuclear plants and how when things go wrong the whole area becomes inhabitable. I have been trying to understand certain things but i am not sure. I hope my understanding of it are not filled with self-inflicted misconceptions. 1) Uranium crystals found in nature...- cshum00
- Thread
- Effects Nuclear Radioactivity Reactions
- Replies: 3
- Forum: High Energy, Nuclear, Particle Physics
-
S
Electrolytic Reactions: Anode vs Cathode
hey guys i have put this question to clarify some points on battery anode cathode reactions...let me describe this..if we consider the cell to be two electrodes with in between electrolyte .. at anode one has the situation: metal=metal ion+electron released i.e oxidation takes place..! so...- shivaniits
- Thread
- Anode Cathode Reactions
- Replies: 13
- Forum: Chemistry
-

March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure
Author: Jerry March, Michael B. Smith Title: March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure Amazon Link: https://www.amazon.com/dp/0471720917/?tag=pfamazon01-20 Prerequisities: High-School Chemistry Contents: User comments...- Greg Bernhardt
- Thread
- advanced Chemistry Organic Organic chemistry Reactions Structure
- Replies: 2
- Forum: Science and Math Textbooks
-
S
Mean Free Path and Reactions (Atmospheric Chemistry)
Hi, I have an exam tomorrow for atmospheric chemistry and I was just going over some past papers. In one it asks the following question, "What is meant by "Mean Free Path"? How is this relevant to atmospheric chemistry. My answer was as follows "The mean free path is the average distance a... -
B
Mass loss in common chemical reactions?
In any common chemical reaction that releases energy, say the reaction 2H2 + O2 = 2H2O, what mass is converted to energy via the E = MC2 equation? What sub-atomic particles are converted to energy during ordinary chemical reactions? I was taught us in HS and under-grad chemistry classes that... -
J
Acids and Bases and Their Reactions
Homework Statement Oxalic acid, H2C2O4, which is found in certain pants, can provide two hydronium ions in water. Write balanced equations to show how oxalic acid can supply one and then a second H3O+ ion. Homework Equations There's not really an equation, and if there is please don't make...- jbmiller
- Thread
- Acids Acids and bases Bases Reactions
- Replies: 6
- Forum: Biology and Chemistry Homework Help
-
C
Endothermic Reactions: Activation Energy & Heat Transfer
every reaction requires its reactants to have enough actvation energy in order to start the chemical reaction.sometimes, extra energy is provided in the form of heat. so the reactants take in heat from the surroundings and have enough energy to break old bonds and form new bonds. when they break... -
L
Finding the reactions at the supports, 3D model
Homework Statement Hi. See attached, I need to determine the forces in all members. Homework Equations Sum of forces in X, Y & Z all equal 0. Sum of moments in X, Y & Z all equal 0. The Attempt at a Solution I've drawn the reactions at each support, A, B and C in my 2nd attached...- lazypast
- Thread
- 3d Model Reactions
- Replies: 3
- Forum: Engineering and Comp Sci Homework Help
-
N
Does ΔG° for reactions vary by temperature?
I thought it was only ΔG which varied by temperature,with the equation ΔG = ΔH - TΔS ? It makes no sense to me why ΔG° (= change of gibbs free energy at standard conditions during a reaction) should vary with temperature, but I've gotten an assignment here to calculate ε° for a reaction at... -
S
Chemical Reactions of Substances & Professors' Advice
[1] Good afternoon anyone! I am a Petroleum Engineering student at Palawan State University and conducting a research. I have seen a topic of research, and it is: The Production of Petrol from Air and Electricity, and I need to find some reaction equations for my topic, here are some: Air...- serendipity21
- Thread
- Chemical Chemical reactions Homemade Reactions
- Replies: 21
- Forum: Chemistry
-
K
Why Does Cold Water Increase Survival Rates in Drowning Incidents?
People who have been submerged in very cold water, and presumed drowned, have sometimes been revived. By contrast, people who have been submerged for a similar period of time in warmer water have not survived. Suggest reasons for this difference. I know this has to do with the fact that... -
L
Different kinds of reactions What are the nonreactive cmpds there for ?
There are many different kinds of reactions, but it seems evey one of them has these extra compounds on the reaction arrow (→) that don't even do anything... They say they are catalysts or whatever, but what are they even doing? For instance, I was writing out what the product is from...- Lo.Lee.Ta.
- Thread
- Reactions
- Replies: 1
- Forum: Chemistry
-
T
Fission/Fusion reactions confusion help
Fission/Fusion reactions confusion help please Homework Statement http://www.xtremepapers.com/papers/CIE/Cambridge%20International%20A%20and%20AS%20Level/Physics%20%289702%29/9702_s03_qp_4.pdf number 6...- thoradicus
- Thread
- Confusion Reactions
- Replies: 1
- Forum: Introductory Physics Homework Help
-
M
Why do tosylates conversions only happen as bimolecular reactions?
When an ROH is converted to an R--OTs, why must this mechanism be either Sn2 or E2?- member 392791
- Thread
- Reactions
- Replies: 2
- Forum: Chemistry
-
A
Understanding precipitation reactions
"When the precipitating agent is added to the solution it causes it to become supersaturated. This starts the process of nucleation where ions and molecules will clump together to form small particles. A small amount of nucleation is necessary to start precipitation. However as the reaction... -
E
Weird Statics Truss Problem - Finding reactions
So I'm looking for the reaction forces at the left end of the truss before I solve for the forces in the members of the truss in terms of F and theta. I summed forces in the x, y direction, took moments about point A,B, to get 4 equations in 4 unknowns (Ax, Ay, Bx, By). However, although Ax,Bx...- eurekameh
- Thread
- Reactions Statics Truss Weird
- Replies: 11
- Forum: Engineering and Comp Sci Homework Help
-
A
A question on precipitation reactions
My chem textbook says that in precipitation reactions you lower the pH of the reaction in order for the precipitate to form quantitatively. It doesn't elaborate at all. What is the meaning of precipitate forming quantitatively?- ASidd
- Thread
- Precipitation Reactions
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
N
Calculate the Support Reactions at A and B as a Result of the Force
Homework Statement The load per foot of beam length varies as shown. For x = 17 ft, the unit load is 373 lb/ft. At x = 0, the load is increasing at the rate of 41 lb/ft per foot. Calculate the support reactions at A and B. The reactions are positive if upward, negative if downward. I have...- Northbysouth
- Thread
- Force Reactions Support Support reactions
- Replies: 2
- Forum: Engineering and Comp Sci Homework Help