Wavelength Definition and 1000 Threads
-
A
Why does the angle of light through a prism change based on its wavelength?
Just a general question on the EM spectrum. angle deviation of the light wave as it passes through a prism seems to be inversely related (looks like a 1/x graph) to its wavelength. So my question, why? My guess is that the deflected path is shorter than the undeflected by cos A, where 0...- Ambidext
- Thread
- Intensity Wavelength
- Replies: 1
- Forum: Other Physics Topics
-
D
Calculate Resonance Wavelength?
Calculate Resonance Wavelength?? Homework Statement Ive been asked to calculate the resonance wavelength of gas in a resonance tube. What I have to calculate this with is the frequency f and the average difference of length between each 1⁄2 wavelength measurement. It says calculate it using...- drc754
- Thread
- Resonance Wavelength
- Replies: 2
- Forum: Introductory Physics Homework Help
-
B
Calculating Wavelength with Wave Speed and Period | Physics Homework Help
Homework Statement What is the wavelength λ of a wave that has a speed of 160 m/s and a period of 4.9 ms? Homework Equations λ = vT The Attempt at a Solution λ = 160 x 4.9 = 784m However this answer is showing up as inccorect when I submit it online. What am I doing wrong?- bfitzp
- Thread
- Wavelength
- Replies: 5
- Forum: Introductory Physics Homework Help
-

De Broglie's wavelength, what is it?
From an exercise I calculated the de Broglie's wavelength of a bullet (mass=40g and speed =1000m/s) to be very small: 1.65651 \times 10 ^{-35}m. What does this mean? That to observe some interference pattern if I throw up these kind of bullets into a double slit, I'd need a width of the slit...- fluidistic
- Thread
- Wavelength
- Replies: 12
- Forum: Quantum Interpretations and Foundations
-
X
Finding the De Broglie Wavelength of a Hydrogen Atom at Room Temperature
Question: Calculate the De Broglie wavelength for a hydrogen atom at room temperature (300K). So far, the only equation I know/have used for De Broglie wavelength is lambda=h/(mv). However, I am not exactly sure how to incorporate the information that the hydrogen atom is at room temperature...- xregina12
- Thread
- Wavelength
- Replies: 1
- Forum: Introductory Physics Homework Help
-
J
Find wavelength for a standing wave
Homework Statement Two sinusoidal waves with identical wavelengths and amplitudes travel in opposite directions along a string with a speed of 10 cm/s. If the time interval between instants when the string is flat is 0.50 s, what is the wavelength of the waves? Homework Equations v = w/k =...- jwxie
- Thread
- Standing wave Wave Wavelength
- Replies: 1
- Forum: Introductory Physics Homework Help
-
B
Wavelength and destructive interference
Homework Statement In the figure the two speakers are producing identical sound waves. The solid lines represent constructive interference regions and the dashed lines represent destructive interference regions. The point labeled 1 is 662.15 m from the bottom speaker and 742.9 m from the...- boogiepicker
- Thread
- Destructive interference Interference Wavelength
- Replies: 1
- Forum: Introductory Physics Homework Help
-
S
Finding wavelength of sound waves
Homework Statement The two speakers are producing identical sound waves. The solid lines represent constructive interference regions and the dashed lines represent destructive interference regions. The point labeled 4 is 1228.5 m from the bottom speaker and 1618.5 m from the top speaker...- SilentBlade91
- Thread
- Sound Sound waves Wavelength Waves
- Replies: 8
- Forum: Introductory Physics Homework Help
-
R
Help finding de Broglie wavelength for an electron
Homework Statement An electron in the first Bohr orbit of a hydrogen atom (a_0=5.3*10^-11m) has a KE of 13.6 eV. Express the de Broglie wavelength for this electron in multiples of the atomic circumference. Homework Equations lambda=h/p =h/(sqrt(2mqV) The Attempt at a...- reality99
- Thread
- De broglie De broglie wavelength Electron Wavelength
- Replies: 1
- Forum: Advanced Physics Homework Help
-
A
How Can You Measure Light Wavelengths Using Microscope Slides and Rubber Bands?
In school we somehow measured the minimum and maximum wavelength our eyes could see Unfortunately, all I remember is that we were looking through a slit between jaws of some sort of calipers and used a ruler to do measurements Can anyone please give me an insight of how to set up such... -
V
Very simple problem about wavelength, just a double check on work
Homework Statement radiation with a frequency of 1200cm^-1, calculate the wavelength in meters and the frequency in hertz Homework Equations wavenumber = 1/λ freq = c/λ The Attempt at a Solution 1200cm^-1 = 1/λ λ = 8.33*10^-6 m freq = (3.0 * 10^8)/(8.66*10^-6)...- vande060
- Thread
- Wavelength Work
- Replies: 4
- Forum: Introductory Physics Homework Help
-
M
Calculating the 'Wavelength' Of the first harmonic
Homework Statement Hi, i have a problem that I'm trying to figure out: An open ended tube has a resonant frequency of 261.6Hz, and the speed of sound is 343m/s. What is the tubes' length?Homework Equations v=(f)(λ) v=(f)(λ/2) The Attempt at a Solution calculating with the equation v=(f)(λ)...- mHo2
- Thread
- Harmonic Wavelength
- Replies: 4
- Forum: Introductory Physics Homework Help
-
V
Wave Problem - Amp, Freq, Vel & Wave Length
Homework Statement The displacement function for a string carrying a transverse wave is y(x, t) = 2.0mm*sin(20*x/m − 600*t/s) Determine the amplitude, frequency, velocity and wavelength of the wave. Determine the maximum transverse speed of any point on the string. Homework...- vande060
- Thread
- Amplitude Frequency Velocity Wave Wavelength
- Replies: 3
- Forum: Introductory Physics Homework Help
-
L
Gravity Wave - Wavelength / Freq
Hi Folks Does anyone know if Gravity waves have been measured accurately enough to have a wavelength and frequency at the quantum scale. I'm looking for any data measured in Plancks. Bit of a random one, it relates to some research I am doing at the moment. Thanks Best Colin- lowing99
- Thread
- Gravity Wave Wavelength
- Replies: 8
- Forum: Other Physics Topics
-
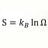
E = Em sin(kx-wt) and I = T+R what wavelength is reflected wave
When a light wave enters a medium the electric field value of the wave is smaller in the medium. With the incident wave = transmission wave + reflected wave. If the E field of the reflected wave is known . Can the wavelength of the reflected wave be obtained from these relationships : E = Em... -
P
Quantum, photon, wavelength relationship
Hi Guys, Is there a SIMPLE (I am not a physicist) relationship between a quantum of energy, the photon(s) involved and the wavelength (or frequency) of the photon(s) involved in making up of the quantum? Regards. Pierre- Pierre007080
- Thread
- Photon Quantum Relationship Wavelength
- Replies: 25
- Forum: Quantum Physics
-
M
Finding the de Broglie wavelength from momentum
Homework Statement What is the de Broglie wavelength of a neutron traveling with a momentum equal to 300 \frac{\text{MeV}}{\text{c}}? Homework Equations \lambda = \frac{h}{p} The Attempt at a Solution p = \frac{300 \cdot \left( \left(1\times10^6 \right) \times...- Matty R
- Thread
- De broglie De broglie wavelength Momentum Wavelength
- Replies: 3
- Forum: Introductory Physics Homework Help
-

Confused about wavelength (basic)
I'm confused about how to think about wavelength for something physical, like a simple pendulum or harmonic oscillator. I understand graphically what wavelength is but can't seem to come up with a physical description. I was thinking about ocean waves and that wavelength could be the direct...- mishima
- Thread
- Confused Wavelength
- Replies: 7
- Forum: Other Physics Topics
-
D
Finding the wavelength of this wave
Homework Statement Okay, here is a photo of the wave I did on a graphing program: http://img221.imageshack.us/i/wavelengthsgraph.jpg/ Now the total distance the wave travels is 4.8 M. There are 3 crests and 3 troughs, but there is only 2 wavelengths throughout the wave. This confuses me...- -Dragoon-
- Thread
- Wave Wavelength
- Replies: 6
- Forum: Introductory Physics Homework Help
-
O
Calculate wavelength of electron
Homework Statement http://www.screencast.com/users/trinhn812/folders/Jing/media/9582a1aa-b21a-4e50-8774-b60866a7d666 Homework Equations lambda=h/p The Attempt at a Solution So I got 4.04E-12 m but the book says the answer is 3.23pm I'm not sure where I'm wrong- okgo
- Thread
- Electron Wavelength
- Replies: 3
- Forum: Introductory Physics Homework Help
-
F
Wavelength of photon and size of imaged object
I have read the statement that 'the wavelength of the photons used in imaging an object needs to be much larger than the object itself', although I have never seen/heard a reason explained or the name of a theorem quoted. I have seen this in the description of more than one imaging modalities...- fred1234
- Thread
- Photon Wavelength
- Replies: 7
- Forum: Quantum Physics
-
S
Work function of silver given wavelength
Homework Statement If electrons can be ejected from a silver surface using light with wavelengths as large as 262 nm. What is the work function for silver? Homework Equations work function=hf c=f\lambda The Attempt at a Solution Given the wavelength, I solved for f =...- sheepcountme
- Thread
- Function Silver Wavelength Work Work function
- Replies: 2
- Forum: Introductory Physics Homework Help
-
T
Work function of a cathode, when the wavelength of light is increased by 50%
Homework Statement The maximum kinetic energy of photoelectrons is 3.14 eV. When the wavelength of the light is increased by 50.0 %, the maximum energy decreases to 1.11 eV. What is the work function of the cathode? Homework Equations Kmax = E elec - E not = hf - hfnot hf =...- Tina20
- Thread
- Cathode Function Light Wavelength Work Work function
- Replies: 5
- Forum: Introductory Physics Homework Help
-
U
De Broglie wavelength and mass
I've been studying quantum physics for a little over a semester now and I'm having trouble wrapping my head around the physical significance of matter waves. The derivation also confuses me because it states the momentum of particle with mass as mc, which is only true for a massless photon. I...- unchained1978
- Thread
- De broglie De broglie wavelength Mass Wavelength
- Replies: 2
- Forum: Quantum Interpretations and Foundations
-
R
Maximum detectable wavelength of a photoresistor
to increase maximum detectable wavelength of a photoresistor which is equal to planck's constant/ semiconductor energy gap , we need to decrease the energy gap only planck's constant cannot be increased or decreased, right?- Rula
- Thread
- Maximum Photoresistor Wavelength
- Replies: 2
- Forum: Other Physics Topics
-
A
De Broglie Wavelength - Can a Person Move Slow Enough?
Just a random thought: is it not possible for a person to move slow enough to have an observable de Broglie wavelength? For example a person moving at around 10^-30 m/s should have a wave nature that is very observable.- Arctic
- Thread
- De broglie De broglie wavelength Wavelength
- Replies: 4
- Forum: Quantum Interpretations and Foundations
-

Radio communicattion in lower wavelength
Hi community. Once again, a stupid question: if radio waves are electromagneetic waves, generating an electric field in the antenna, then why can't one just shine visual light (also electrromagnetic waves) on same antenna and use that for communication? thanks s- Seanskahn
- Thread
- Radio Wavelength
- Replies: 1
- Forum: Classical Physics
-
M
X-ray Diffraction, Intensity vs. Wavelength Graph
Homework Statement http://b.imagehost.org/0607/Question_7.png Homework Equations [PLAIN][PLAIN]http://d.imagehost.org/0813/Untitled_6.jpg The Attempt at a Solution I was able to get question b, which ends up being 73pm, but as for questions a and c, I was unable to come up with...- Mxbn0
- Thread
- Diffraction Graph Intensity Wavelength X-ray X-ray diffraction
- Replies: 4
- Forum: Introductory Physics Homework Help
-
F
How is the wavelength of monochromatic light measured
how is the wavelength of monochromatic light measured by using diffraction grating and spectometer?- franklinear
- Thread
- Light Wavelength
- Replies: 3
- Forum: Introductory Physics Homework Help
-
P
Finding deBroigle wavelength of a charged electron
Homework Statement Given an electron with total energy 1 KeV, determine it's deBroigle wavelength. Homework Equations E^2 = (mc^2)^2+(pc)^2 \lambda = \frac{h}{p} The Attempt at a Solution (pc)^2 = E^2 - (mc^2)^2 <> p = ± \frac{1}{c} \sqrt{E^2-(mc^2)^2} What am I doing...- ParrotPete
- Thread
- Charged Electron Wavelength
- Replies: 1
- Forum: Advanced Physics Homework Help
-
M
Wavelength of photon in a medium
A photon goes from vacuum into a medium. This causes its momentum to reduce. Hence by the de Broglie formula, its wavelength should increase. But, since the refractive index of the medium is greater than 1, and n_2/n_1 = \lambda_1 / \lambda_2 , and hence, its wavelength should decrease... -
N
How Do Wi-Fi and Bluetooth Devices Transmit Digital Data?
Wi-Fi Wavelength?? First of all,, HI, everybody, I am Sonics from India I was searching on Wi-Fi modems and how other bluetooth enabled or wireless things work. I know that they catch some frequency and then convert it to something that is been sent on that wavelength but I don't know that how...- niksng
- Thread
- Bluetooth Wavelength
- Replies: 1
- Forum: Other Physics Topics
-
S
What is the Correct Wavelength of Light in an Interference Experiment?
Homework Statement Monochromatic light from a point source illuminates two parallel, narrow slits. The centres of the slip openings are 0.8mm apart. An interference pattern forms on a screen placed parallel to the plane of the slits and 49 cm away. The distance between two adjacent dark...- stphillips
- Thread
- Light Wavelength
- Replies: 6
- Forum: Introductory Physics Homework Help
-
S
Q : Verify Wien's displacement law (frequency and wavelength form)?
HINT: SUB in (x=hv/k(b)T) I don't understand How I am supposed to approach this question or how I am supposed to verify it- sam_021
- Thread
- Displacement Form Law Wavelength
- Replies: 1
- Forum: Advanced Physics Homework Help
-
C
Wavelength and Extinction Coefficients
I have been researching on the net and books for some time now. I have a few tables and equations but was wondering if anyone has the one I am looking for? I'm looking for a table that lists Wavelength(nm), Extinction Coefficient(k), Absorption Coefficeint(a) and Density(d) values from ~300nm...- Chad Stoltzma
- Thread
- Coefficients Extinction Wavelength
- Replies: 2
- Forum: Atomic and Condensed Matter
-
E
Math of De-Broglie wavelength boggles me
According to simple math you get \lambda=h/p for photons. and the assumption is for everything else, whilst for everything else which is NOT restmass=0 it should be \lambda=hc/E. So why is it referred to as simple \lambda=h/p when clearly it is not true mathematically. It would be true for...- etamorphmagus
- Thread
- Wavelength
- Replies: 8
- Forum: Quantum Physics
-
I
Sub woofers and tweeters wavelength
[b]1. question [b]2.hello Dear , I have a question for you please , When you buy a home cinema system, you can place the subwoofer almost anywhere, even behind your couch. But the tweeters (speaker for high frequencies) must be placed very accurately. Explain why! Hint: use...- Istfan
- Thread
- Wavelength
- Replies: 2
- Forum: Introductory Physics Homework Help
-
X
How do we measure the wavelength of electromagnetic waves?
How the wavelength of wave is measured? My idea of electromagnetic wave is there is an invisible line traveling through space moving up and down like a sine wave we see on CRO. Is this idea correct? Or is it the the variation in electric and magnetic field of the wave that we relate this...- x+iy
- Thread
- Wavelength
- Replies: 9
- Forum: Other Physics Topics
-
B
Modern Physics I: Find Energy Loss & Wavelength
Homework Statement An electron with energy 10^3 MeV collides with a photon whose wavelength corresponds to the maximum wavelength of the 2.7-K cosmic background radiation. What is the maximum energy loss the electron can suffer as a result of the collision? What is the wavelength of the photon...- baltimorebest
- Thread
- Energy Energy loss Loss Modern physics Physics Wavelength
- Replies: 13
- Forum: Advanced Physics Homework Help
-

How Do Transition Wavelengths Compare Between Hydrogen and Helium?
6. http://i111.photobucket.com/albums/n149/camarolt4z28/2010-09-23195548.jpg?t=1285299756 I'm thinking I use the omegaij formula to determine the frequency between energy levels and then use that to calculate the wavelength...- Shackleford
- Thread
- Compare Transition Wavelength
- Replies: 3
- Forum: Advanced Physics Homework Help
-
F
Wavelength, emission spectrum, electron transitions
Homework Statement Determine the wavelength (in nm, to one decimal place) of the line in the emission spectrum of the He+ ion produced by a transition from n = 3 to n = 1. Homework Equations I used the Rydberg equation for this, although I'm not sure it is the correct one to use...- FsLiu
- Thread
- Electron Emission Emission spectrum Spectrum Wavelength
- Replies: 2
- Forum: Biology and Chemistry Homework Help
-
A
Can Particles in Extreme Conditions Exhibit Quantum Degeneracy?
Homework Statement By estimating the De Broglie wavelength and the inter-particle spacing, find whether the following systems of particles are degenerate: (i) a gas of neutrons in a neutron star with mass density 10^17 kg/m^3 at the temperature of 10^8K (ii)a gas of oxygen molecules at...- Anabelle37
- Thread
- De broglie De broglie wavelength Wavelength
- Replies: 1
- Forum: Advanced Physics Homework Help
-

Calculating De Broglie Wavelength from Kinetic Energy
Homework Statement Show that the de Broglie wavelength of a particle of mass m and kinetic energy KE is given by \lambda = \frac{hc}{\sqrt{KE(KE + 2mc^2)}} Homework Equations The Attempt at a Solution I know: KE = {\gamma}mc^2 - mc^2 = pc - mc^2 But from here I am...- jinksys
- Thread
- De broglie De broglie wavelength Wavelength
- Replies: 1
- Forum: Advanced Physics Homework Help
-
P
Energy vs wavelength for a photon in GR
If a theoretical single photon followed a geodesic toward a large mass in space, I understand that the wavelength would shorten as it approached the mass. How would the energy be conserved within the photon, because the frequency must surely remain the same?- Pierre007080
- Thread
- Energy Gr Photon Wavelength
- Replies: 5
- Forum: Special and General Relativity
-
E
Why is wave diffraction around object better at longer wavelength?
Wikipedia: Does the wave theory itself (the mathematical formulation) imply that diffraction is more efficient at longer wavelengths? I just want the logical connection of why (for example) radio waves diffract and bend around objects better than visible light. :confused: Thank you.- etamorphmagus
- Thread
- Diffraction Wave Wavelength
- Replies: 16
- Forum: Optics
-

Find the wavelength of the particle
Homework Statement I'm not even sure the problem is about matterwave: A particle of mass m and charge e is accelerated by a potential difference V. Find the wavelength of the particle. Show that this result agrees with the classical one when the non relativistic limit is taken.Homework...- fluidistic
- Thread
- Particle Wavelength
- Replies: 4
- Forum: Introductory Physics Homework Help
-

What is the minimum wavelength of x-rays produced by a 50 kV emf?
Homework Statement An x-rays tube is connected to a 50 kV emf. Calculate the minimum wavelength that one can find in the radiation spectra that the tube produces.Homework Equations No idea! This is why I ask help here. The Attempt at a Solution Stuck at start. I've searched in google and...- fluidistic
- Thread
- Rays Wavelength X rays
- Replies: 1
- Forum: Introductory Physics Homework Help
-
2
The Atom: wavelength, energy, kinetic energy, etc. of photons
here are the problems that I need help with: http://imgur.com/a/fERMC/1 I am afraid that I know not much information about the questions. Sadly, I am enrolled in a poorly written Physics class online. I am not looking for the answers, but just how to go about finding them. The book never...- 278225mw
- Thread
- Atom Energy Kinetic Kinetic energy Photons Wavelength
- Replies: 1
- Forum: Introductory Physics Homework Help
-
D
Gradient of Wavelength vs Period Graph?
We've got a table of periods (in seconds) and their corresponding wavelengths for creating resonance in a closed pipe. I've been told that plotting a graph of period (on x axis) vs wavelength, and finding the gradient of that linear line will tell me the speed of sound in air. I can do that...- donkeycopter
- Thread
- Gradient Graph Period Wavelength
- Replies: 1
- Forum: Introductory Physics Homework Help
-
D
Calculating Time & Wavelength of 1000Hz Noise
Hi, "Somebody makes a noise with a frequency of 1000Hz. Calculate how long it will take to reach somebody 3m away. What will be the wavelength?" I can't figure out how it fits into any of the formulas I have! Thanks! :)- donkeycopter
- Thread
- Time Wavelength
- Replies: 3
- Forum: Introductory Physics Homework Help