Wavelength Definition and 1000 Threads
-
D
How Does Changing Wavelength Affect the Central Peak in Single-Slit Diffraction?
In my physics book, one of the basic quick quizzes checkpoints asks what happens to the central peak in a diffraction envelope when you decrease the wavelength of light (from 650 nm to 450 nm, for reference). My understanding is that the width of the peak would decrease, while the number of... -
S
Infinite wavelength resonant antennas
Could somebody explain what does mean "infinite wavelength antenna" and what advantages does it have? What is resonant antenna and advantages? http://webcache.googleusercontent.com/search?q=cache:fHdO6P-aoIIJ:dspace.nitrkl.ac.in:8080/dspace/bitstream/2080/1320/1/MMET.pdf+&cd=4&hl=en&ct=clnk&gl=ca- Stanley514
- Thread
- Antennas Infinite Resonant Wavelength
- Replies: 2
- Forum: Electrical Engineering
-
D
De Broglie wavelength of a tennis ball
This is a multiple choice question. The de Broglie wavelength of a moving tennis ball is calculated as 1x10^-33. This means that the moving tennis ball A)Diffracts through a narrow slit. B)Does not behave as a particle C)Does not display wave properties D)Is traveling at the speed of light The...- Davidmb19
- Thread
- Ball De broglie De broglie wavelength Tennis Wavelength
- Replies: 8
- Forum: Introductory Physics Homework Help
-
B
What does Wavelength/degree mean?
Hi If c(velocity)/f(frequency) = wavelength and 1/f(frequency) * 6 = degrees What does Wavelength/degree mean?? Regards- bitencrypt
- Thread
- Degree Wavelength
- Replies: 9
- Forum: Other Physics Topics
-
R
Wavelength Calculation in a Ripple Tank with Varying Speeds
Homework Statement In a ripple tank, you measure the speed of a wave to be 12 cm /s in the deep section and 9.0m/s in the shallow section. If the waves in the deep section that are 11.5 cm long cross over to the shallow section, what would be the wavelength in the shallow section Homework...- rrosa522
- Thread
- Wavelength
- Replies: 1
- Forum: Introductory Physics Homework Help
-
R
If wavelength changes does speed change
Homework Statement if [/B]wavelength changes does speed change (in standing waves) 2. Homework Equations velocity = wavelength * frequency The Attempt at a Solution i think it does because the wavelength changes so the speed will also change[/B]- rrosa522
- Thread
- Change Speed Wavelength
- Replies: 4
- Forum: Introductory Physics Homework Help
-
J
Finding wavelength and intensity of a specific light
Hi people not sure if this is feasible but this is the idea and like to hear your comments i wan 2 shine a specific color of light (could be colored LED, normal light,) to determine the wavelength n intensity of it, so i am thinking of using a photodiode and an opamp connected with a...- johnny1985
- Thread
- Intensity Light Specific Wavelength
- Replies: 22
- Forum: Electrical Engineering
-
Are These Photon Wavelength and Linear Momentum Calculations Correct?
Homework Statement I had to find wavelenght and linear momenta of fotons with energies of 3eV, 50 KeV and 1.0 MeV Are these correct? Homework Equations E=hc/λóλ=hc/E and p= h/ λ The Attempt at a Solution a. 3eV Hence λ=(6.63E-34*3E8)/3=6.63E-26m p = 6.63E-34/6.63E-26 = 1E-8 b. 50 KeV =...- mss90
- Thread
- Linear Linear momentum Momentum Wavelength
- Replies: 3
- Forum: Introductory Physics Homework Help
-
X
Calculating Average Emission Wavelength from Spectral Intensity Chart
I haven't had calculus in over 2 years and so I am not exactly sure how to go about this. I am inclined to believe some form of integration would be needed. I took the chart below from a paper on LED wavelength emission. The authors say that the vertical line represents the average emission...- Xtensity
- Thread
- Average Emission Intensity Wavelength
- Replies: 9
- Forum: General Math
-
U
Refraction-Speed difference at different wavelengths
Hi all, I've read that when light undergoes refraction into a medium with higher refractive index it changes speed and this is explained by the electrons of the medium absorbing the photon energy, they hold onto it then eventually re-emit the light if the frequency of light doesn't match the... -
X
Troubleshooting Infrared Wavelength Estimation Experiment
1. Homework Statement Hi, I have to do lab experiment - estimating infrared wavelength (from remote control). My experimental setup includes CD, remote control, webcam (without IR filter, so I can see the infrared radiation), sheet of paper (I will see diffracted light spots on it) with hole...- XXZZ
- Thread
- Diffraction Infrared Ir Radiation Remote control Wavelength
- Replies: 3
- Forum: Introductory Physics Homework Help
-

De Broglie Wavelength: Moving Particles & Equations
according to De Brogli wavelength equation not every thing has a wavelength instead everything that moves has a wavelength right?- gracy
- Thread
- Wavelength
- Replies: 11
- Forum: Quantum Physics
-
R
Finding the theoretical value of the wavelength for a double slit experiment?
Homework Statement m=maximum h=distance on the screen from the center of the pattern to the mth maximum D=distance from the central bright peak to the slits d=distance between two slits I have m=1 d(mm)=0.25mm D=2000mm h=0.5mm wavelength= 6.25E-5mm Homework Equations So I used...- ronsa
- Thread
- Double slit Double slit experiment Experiment Physics Slit Theoretical Value Wavelength
- Replies: 1
- Forum: Introductory Physics Homework Help
-

Gamma photon wavelength: Is there a limit?
Is there any known limit to the energy of a photon? I've seen a reference to pair production in the highest bracket over 1.02 MeV and I've seen references to energies from cosmic sources in the TeV range which aren't very well understood but is there any theoretical limit?- jerromyjon
- Thread
- Gamma Limit Photon Wavelength
- Replies: 6
- Forum: High Energy, Nuclear, Particle Physics
-
D
Measuring the Wavelength of Light
How to measure of wavelength of light source?- dr naz chuhan
- Thread
- Light Measuring Wavelength
- Replies: 3
- Forum: Optics
-
K
Wavelength, speed and frequency question
Homework Statement Typical AM radio wavelengths are about as long as a football field, while typical FM radio wavelengths are about a meter long. Which one has the highest frequency? If AM wavelength is 100 times longer than an FM radio wavelength, then how do the frequencies compare? How do...- kirsten_2009
- Thread
- Frequency Speed Wavelength
- Replies: 2
- Forum: Introductory Physics Homework Help
-
J
De Broglie Wavelength of an electron
1. The velocity of the electron in the ground state of the hydrogen atom is 2.6 x 10^8 m/s. What is the wavelength of this electron in meters?2. De Broglie's equation: lamda = h/p p=mvThe Attempt at a Solution ... (6.626 x 10^-34) / (2.6 x 10^8 x 9.11 x 10^-31) = 2.798 x 10^-12 meters This...- Janet
- Thread
- De broglie De broglie wavelength Electron Wavelength
- Replies: 3
- Forum: Introductory Physics Homework Help
-
M
Wavelength uncertainity for particle in a box
For a particle in a box, we have been explained that the wave function is like a standing wave. Then we wrote:- λ = 2L/n where λ is the wavelength of the nth energy state. But a moving particle is considered as a wave packet and so does not have an unique wavelength. Then, how are we determining...- Maharshi Roy
- Thread
- Box Particle Wavelength
- Replies: 3
- Forum: Quantum Physics
-
L
De Broglie wavelength of helium atoms
Homework Statement a) In the double slit interference pattern for helium atoms, the kinetic energy of a beam of atoms is 0.020 eV. What is the de Broglie wavelength of a helium atom with this kinetic energy? b) Also, estimate the de Broglie wavelength of the atoms from the fringe spacing...- leehufford
- Thread
- Atoms De broglie De broglie wavelength Helium Wavelength
- Replies: 2
- Forum: Advanced Physics Homework Help
-
F
Flourescence and self-absorption cause shift in wavelength
Hello Forum, I understand how fluorescence works: there is an absorption spectrum and an emission spectrum. The two spectra are shifted relative to each other in the sense that the absorption peak wavelength is different from the emission peak wavelength (Stokes shift). That said, fluorescent... -
H
De Broglie wavelength - model for comparing photons and electrons
Hello, I'm thinking about the wavelength of a freely propagating photon vs. a freely propagating electron. For the photon, we have the classical picture of oscillating E and B fields perpendicular to the direction of propagation, and we call the wavelength of the photon, which can be...- HJ Farnsworth
- Thread
- De broglie De broglie wavelength Electrons Model Photons Wavelength
- Replies: 1
- Forum: Quantum Interpretations and Foundations
-
N
Linewidths - Frequency and Wavelength
Hi, Probably a really stupid question... but I'm confused about how to relate a line width in frequency to one in wavelength. To me it seems obvious that if there's a broader spread of frequencies, there must be a broader spread of wavelengths, and vice versa - after all, the line is broader...- Naz93
- Thread
- Frequency Wavelength
- Replies: 1
- Forum: Electromagnetism
-
F
Relation of radiation wavelength and photosynthetic photon flux?
Dear all, If I have the value of photosynthetic photon flux in unit [ micro mole per meter square per second] as an output for ultra violet sensor. How can I know the corresponding wavelength of that radiation ? and can I know from that wavelength what is the type of the ultraviolet...- Farah
- Thread
- Flux Photon Radiation Relation Wavelength
- Replies: 3
- Forum: Quantum Physics
-
A
Electromagnetics - finding wavelength from e_r and f
Homework Statement A transmission line is 1 meter and has a relative dielectric constant of 2.25 Find the phase velocity u and the wavelength λ for a frequency of 50 MHz. Homework Equations λ=?(ε_r, f) u=λf The Attempt at a Solution I am unable to get from the relative...- a_lawson_2k
- Thread
- Electromagnetics Wavelength
- Replies: 2
- Forum: Engineering and Comp Sci Homework Help
-
R
Wavelength of 1.5keV (kinetic energy) electron
How would one find the velocity of this electron. Is it considered relativistic or will 1/2mv^2 work just fine??- rem45
- Thread
- Electron Energy Kinetic energy Wavelength
- Replies: 1
- Forum: Electromagnetism
-
S
Are the wavelength of standing wave and sound wave produced same?
Homework Statement a string is tightened at 2 ends. the string is then plucked , a standing wave is produced. are the wavelength of standing wave and sound wave produced same? Homework Equations The Attempt at a Solution- somecelxis
- Thread
- produced Sound Sound wave Standing wave Wave Wavelength
- Replies: 3
- Forum: Introductory Physics Homework Help
-

What determines the wavelength of light an object reflects?
Now correct me if I`m wrong please. So the sun shines is light delivering its spectrum across Earth and objects absorb certain wavelengths and reflect certain wave lengths. The reflected wavelengths is the color we see that object as. So what is it about the object that determines the wavelength... -
D
Wavelength of sound affected by the moving observer?
Homework Statement why the wavelength of sound is affected by the moving observer? because as the observer move, the sound source is still stationary . so in my opinion it's not affected by the moving observer. the ans is C for this question. i can't understand. Homework Equations...- desmond iking
- Thread
- Observer Sound Wavelength
- Replies: 1
- Forum: Introductory Physics Homework Help
-
M
Lasers: Power and wavelength dependence
I have a 1550nm diode laser. I have a function generator sending a triangular function into the fast input of the laser; however, if I am within the bandwidth of the laser (under 10kHz), my output power of the laser mimics my fast input signal, so I get a triangular function on my O-scope with...- MelioraGator
- Thread
- Lasers Power Wavelength
- Replies: 2
- Forum: Optics
-
F
How can the wavelength of blue light be 750nm?
Homework Statement A Visible light is shone on a diffraction grating. Two rays are produced, One Orange ray with 600 nm which is at the fifth order maxima. It coincides with a blue ray at the fourth order maxima. Find the wavelength of the blue light. Homework Equations n x lamda =...- FaroukYasser
- Thread
- Light Wavelength
- Replies: 2
- Forum: Introductory Physics Homework Help
-
T
What happens when a wavelength is longer than the length of coax?
We are all taught that the TEM modes in coax have no low frequency cut-off. So this would suggest that nothing different happens for very long wavelengths that are longer than the coax itself. However, in this rather shocking paper here: http://vixra.org/pdf/1403.0964v5.pdf ...it is suggested...- ThunderStorm
- Thread
- Length Wavelength
- Replies: 5
- Forum: Electrical Engineering
-
M
Balmer Wavelength for Hydrogen-like Fe Atom (Z=26): 0.971 nm
I have been having trouble with this Balmer wavelength problem and was hoping I can get a little guidance. The question: Find the balmer wavelength (n=3 --> n=2) emitted from a hydrogen-like Fe atom (z=26) The answer is supposed to be 0.971 nm My attempt: 1/lambda = R(1/n'^2 - 1/n2)...- Meekay
- Thread
- Wavelength
- Replies: 1
- Forum: Introductory Physics Homework Help
-
N
How hard would it be to output a stable beam if a certain wavelength?
I want to output a specific wavelength of infrared light, I believe the wavelength was 9.25nm, how hard would that be and how would I proceed?- Nerdydude101
- Thread
- Beam Hard Output Stable Wavelength
- Replies: 7
- Forum: Other Physics Topics
-
D
Diffraction - opening smaller than wavelength
Hi! I have this problem: Homework Statement Which combination will produce the least degree of diffraction? A. λ of 2.0m through an opening of 1.0 cm B. λ of 30m through an opening of 2.0 m C. λ of 2.0m through an opening of 25 m D. λ of 5.0nm through an opening of 45 m The Attempt...- dragon-kazooie
- Thread
- Diffraction Wavelength
- Replies: 1
- Forum: Introductory Physics Homework Help
-
N
Finding initial wavelength of photo given angle of deflection
Homework Statement Find the initial wavelength of a photon that loses half its energy when it Compton-scatters from an electron and emerges at 90∘ to its initial direction of motion. The Attempt at a Solution Ei = Ef +KE Ei = 0.5Ei + KE Using compton's formula for scattering...- negation
- Thread
- Angle Deflection Initial Photo Wavelength
- Replies: 3
- Forum: Introductory Physics Homework Help
-
P
Bremsstrahlung wavelength range
i need to calculate what percentage of the energy put into a X-ray tube will be between the minimum wavelength and the longest wavelength suitable for it's application (as to calculate how efficiently it does it's job) with only the input voltage (unless the anode material is important) the...- pixelpuffin
- Thread
- Bremsstrahlung Range Wavelength
- Replies: 7
- Forum: High Energy, Nuclear, Particle Physics
-
J
Best way of measurement sodium wavelength?
Hello everybody ,A friend of mine told me that best way (The most accurate) to do this is grating diffraction experiment , What u think? Thanks in advance- justawebuser
- Thread
- Measurement Sodium Wavelength
- Replies: 2
- Forum: Classical Physics
-
J
Wavelength difference of sodium d lines?
Hello everyone , I googled for it but nothing came up, Where can I find that? Thanks in Advance ,- justawebuser
- Thread
- Difference Lines Sodium Wavelength
- Replies: 5
- Forum: Classical Physics
-
M
Electron diffraction - effect of electron wavelength
Hello, I know how electron diffraction works, and that if you decrease the wavelength of the electrons less diffraction occurs, so the rings are smaller, however was wondering what happens if the wavelength is increased? Is it just the opposite? More diffraction so larger rings? I was also...- Molly1235
- Thread
- Diffraction Electron Wavelength
- Replies: 1
- Forum: Introductory Physics Homework Help
-
De Broglie wavelength computable by just fixing speed of light ?
Hi, I was wondering if it is possible to compute e.g. bohr radii for a metric system whose correspondence to real units is unknown and only the speed of light is known. Let's say the only thing I know is that lightwaves travel x spaceunits in t timeunits, therefore defining the speed of...- xortdsc
- Thread
- Computable De broglie De broglie wavelength Light Speed Speed of light Wavelength
- Replies: 2
- Forum: Quantum Interpretations and Foundations
-
S
Is Wavelength Doubling Laser a Real Technique?
I've heard of frequency doubling but is wavelength doubling a thing? If so, how efficient?- sparkle_pony
- Thread
- Laser Wavelength
- Replies: 1
- Forum: Optics
-
A
Mechanistic explanation of de Broglie wavelength
I am not proposing a particular explanation and just in case you suspect I have something in mind, I must tell you that I don't. I was wondering if anybody here has some ideas. I understand that you may have objections to my question. One of them might be that a mechanistic model would not work...- alexepascual
- Thread
- De broglie De broglie wavelength Explanation Wavelength
- Replies: 7
- Forum: Quantum Interpretations and Foundations
-

Calculating the temperature of the sun using wavelength of iron atoms
I had this question given in my class A spectral line from iron (mass 55.934 amu) atoms in the sun is measured as 637.4 nm. It's width is measured to be 0.053 nm. Calculate the temperature of the sun?Now I done this using a formulae I was given but it yielded an answer no where near 5778KAbove...- KiNGGeexD
- Thread
- Atoms Iron Sun Temperature The sun Wavelength
- Replies: 11
- Forum: Introductory Physics Homework Help
-
S
Steps per wavelength in mesh density
Hi Could anyone explain to me the concept and significance of mesh density as a function of steps per wavelength? I am asking this in context of a frequency domain EM-solver. Thanks- shpongle
- Thread
- Density Mesh Per Wavelength
- Replies: 3
- Forum: Other Physics Topics
-
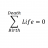
Wavelength with just enough energy
Homework Statement The work function for chromium is ##7.0 \times 10^{-19} J##. What is the wavelength of the light that has just enough energy to cause electrons to be emitted from chromium metal? What is the work function of chromium, in kJ/mol?Homework Equations The Attempt at a Solution...- STEMucator
- Thread
- Energy Wavelength
- Replies: 8
- Forum: Introductory Physics Homework Help
-

Photoelectric Effect - Maximum Wavelength
Hi, I am currently revising photoelectric effect, and i have this question: A metal surface at zero potential emits electrons from its surface if light of wavelength of 450 nm is directed at it but not if light of 650nm is used. Explain why photoelectric emission happens with light of...- LotusTK
- Thread
- Maximum Photoelectric Photoelectric effect Wavelength
- Replies: 1
- Forum: Introductory Physics Homework Help
-
F
De Broglie Wavelength Thought Problem
Homework Statement A) Find the wavelength of an Ewok that has a mass of 50kg and is Running on Edor where 'h' id 1000Js at a velocity of 0.5m/s Yes the value of h to be used is 1000. B) What would the implications of him running be?Homework Equations \lambda=\frac{h}{p}The Attempt at a...- FaraDazed
- Thread
- De broglie De broglie wavelength Wavelength
- Replies: 6
- Forum: Introductory Physics Homework Help
-
P
Calculate Longest & Shortest Wavelength?
Electrons accelerated by a potential difference of 13.14 V pass through a gas of hydrogen atoms at room temperature. A) Calculate the wavelength of light emitted with the longest possible wavelength. B) Calculate the wavelength of light emitted with the shortest possible wavelength. I've...- P1nkButt3rflys
- Thread
- Wavelength
- Replies: 1
- Forum: Introductory Physics Homework Help
-
P
Why in Huygens–Fresnel, Point source should be as small as wavelength?
In Huygens–Fresnel principle, to configure the diffraction pattern after an aperture, we need to consider each point on aperture as a point source of light. I need to make a simulation for diffraction. Here I will need to introduce the size of each point source. my question is why the size of...- payam
- Thread
- Point Source Wavelength
- Replies: 5
- Forum: Other Physics Topics
-

Last scatter by e-, light looks hotter, cooler, time, wavelength.
Over what approximate time frame does 90% (most) of last scattering occur, minutes, seconds, fraction of a second? How does this time frame relate to the period of the gravitational radiation? If the period of gravitational radiation were short it would washout b-modes? Thanks for any...