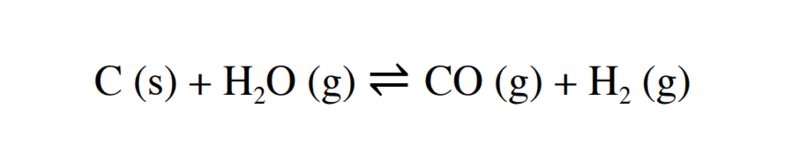samy4408
- 62
- 9
Hello we learned about the chemical equilibrium and how to write it's formula in the case of liquid and gaseous phase , what about a reaction involving different phases ? like this one : how do we write the formula for the chemical equilibrium ? do we just ignore the carbon ,is there any rules to write the chemical equilibrium that i forgot ?