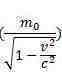- #1
manifested
- 2
- 0
If the energy of single photon of electromagnetic radiation is defined by its wavelength, could it also be set equal to its potential and kinetics?(I’m probably missing something)
Please check this statement:

Where :
h is planks constant
f is frequency.
m0 is rest mass
M is the relativistic mass
v is velocity
If rest mass of electron is zero then mc2 is zero therefore the total energy must be all kinetic(right?).
The only way I could see the kinetic energy not being zero is if somehow the relativistic mass is not zero…or maybe I got it completely wrong…?
Thx in advance.
Please check this statement:
Where :
h is planks constant
f is frequency.
m0 is rest mass
M is the relativistic mass
v is velocity
If rest mass of electron is zero then mc2 is zero therefore the total energy must be all kinetic(right?).
The only way I could see the kinetic energy not being zero is if somehow the relativistic mass is not zero…or maybe I got it completely wrong…?
Thx in advance.