vcsharp2003
- 913
- 179
- TL;DR Summary
- Why is the time interval of collision equal to time taken between successive collisions with the wall?
I have been trying to make sense of the derivation of pressure under Kinetic Theory of Gases chapter, but it's not making sense to me when the impulse momentum equation is used for the collision between a gas molecule and the wall of the container.
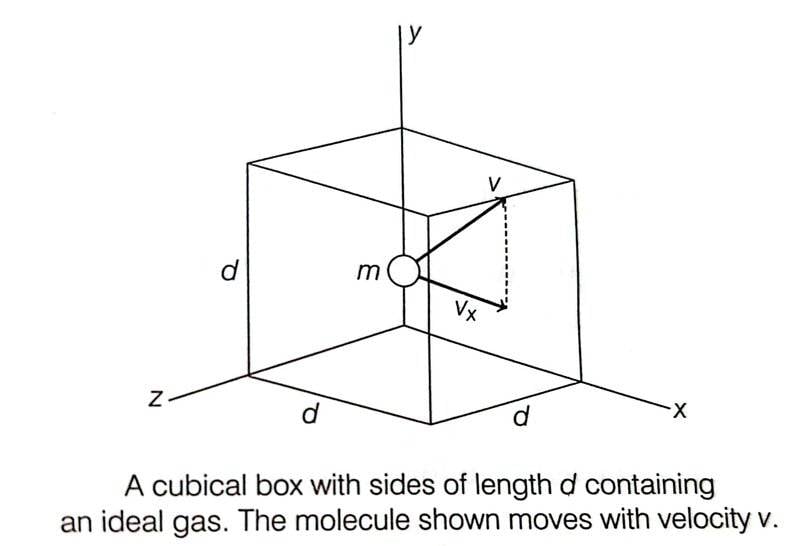
The book says that for the elastic collision between a gas molecule and the wall, ##\Delta p_x = -mv_x- mv_x=-2mv_x## where ##\Delta p_x## is the change in momentum of the gas molecule after colliding elastically with the wall. This makes sense, but the next step seems incorrect to me.
The book says that ##\Delta t = \dfrac {2d}{v_x}## for the equation ##F \Delta t = \Delta p_x##. This seem confusing since the ##\Delta t = \dfrac {2d}{v_x}## is the time for the gas molecule to travel to the opposite wall and back to the original wall i.e. time between two successive collisions with the same wall. According to my knowledge, ##\Delta t## should be the time interval of the collision between gas molecule and the wall ( i.e. time interval of contact during collision).
Could someone please explain why we're not taking the time interval of collision but the time between collisions for ##\Delta t##?
The book says that for the elastic collision between a gas molecule and the wall, ##\Delta p_x = -mv_x- mv_x=-2mv_x## where ##\Delta p_x## is the change in momentum of the gas molecule after colliding elastically with the wall. This makes sense, but the next step seems incorrect to me.
The book says that ##\Delta t = \dfrac {2d}{v_x}## for the equation ##F \Delta t = \Delta p_x##. This seem confusing since the ##\Delta t = \dfrac {2d}{v_x}## is the time for the gas molecule to travel to the opposite wall and back to the original wall i.e. time between two successive collisions with the same wall. According to my knowledge, ##\Delta t## should be the time interval of the collision between gas molecule and the wall ( i.e. time interval of contact during collision).
Could someone please explain why we're not taking the time interval of collision but the time between collisions for ##\Delta t##?