ProjectFringe
- 96
- 10
Thanks again everyone for your responses to my many questions! 
I think the following steps could have a small probability of success in creating the compound I want. This assumes that this compound is being created in a lab and at an atomic level. Therefore, the only things which can react are what is listed in each step (one atom of each).
I know this is long and difficult to read, but I did my best to explain my thinking as best I could and would appreciate any feedback! Step 1. NH+ forms double bond with 40KN
Step 1. NH+ forms double bond with 40KN
The isotope of potassium used is 40K. 40K is a natural, long-lived radioactive isotope of potassium. The dots represent unpaired electrons, and the numbers represent the number of electrons in the outer shell of each nitrogen. This compound is unbalanced and unstable.
.H+N=NK:
7 8
Step 2. 41Ca+ is added to the original compound, N2KH++ 41Ca+
The isotope of calcium used is 41Ca. 41Ca is a natural, long-lived radioactive isotope of calcium. This compound is balanced, but unstable because 41Ca will naturally decay.
Ca+H+N=NK:
8 8
Step 3. 41Ca+ decays to 40K and H+.
Under normal circumstances, 41Ca usually decays to 41K through electron capture. However, in this case since the cation of 41Ca is being used, only one electron is available in the outer shell of 41Ca+. This electron is strongly attached to the nitrogen, which is now balanced, making it unavailable to the 41Ca+. Therefore, using an electron from an inner shell would result in an undesirable total number of electrons for 41Ca+. For this reason, in this instance, I believe electron capture may not be the best option. However, 41Ca+ still must decay. Other ways of decay, such as neutron activation or beta decay, are either not available or lead to a more unstable state. Therefore, with the energy available from the decay of 41Ca+ and possibly the decay of the current 40K, 41Ca+ removes a proton, creating another 40K, a long-lived radioactive isotope of potassium (even longer-lived than 41Ca) and a free proton (H+). This proton joins the available lone pair, and 40K replaces 41Ca+. This creates the most balanced version of this compound to date. However, it is still unstable because 40K will continually decay.
H+KN=NKH+
8 8
Step 4. The original 40K decays to 40Ca through beta decay
The original 40K has been naturally decaying to 40Ca. However, with the creation of 40Ca, another electron is now available. Therefore, the 40Ca unbalances the formerly balanced compound.
H+KN=NH+ Ca
8 9
Step 5. 40Ca decays to 41Ca+ through neutron activation
The decay of 40Ca to 41Ca is a natural process, under the right conditions, even though 40Ca is stable. Similarly, in this case, the desire to lose the extra electron (created from the decay of 40K to 40Ca) causes 40Ca to use this extra electron, in combination with the available proton (H+), to decay to 41Ca+. This occurs through, what would basically be, neutron activation. This process rebalances the compound, creating essentially the same compound as in step 2.
H+KN=NCa+:
8 8
It also allows for a continuous cycle of steps 2 through 5, creating reflections of itself as it passes through a neutral state.
Step 5 (or 2) > Step 3 > Step 4 > Step 5 (or 2) > Step 3 > Step 4 > back to the beginning
H+KN=NCa+: > H+KN=NKH+ > CaH+N=NKH+ > :Ca+N=NKH+ > H+KN=NKH+ > H+KN=NH+Ca > back to the beginning
+ > N > - > - > N > + > back to the beginning
Below is an image of the above because I couldn't get the spacing right
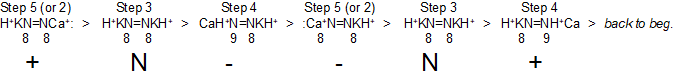
I think the following steps could have a small probability of success in creating the compound I want. This assumes that this compound is being created in a lab and at an atomic level. Therefore, the only things which can react are what is listed in each step (one atom of each).
I know this is long and difficult to read, but I did my best to explain my thinking as best I could and would appreciate any feedback!
 Step 1. NH+ forms double bond with 40KN
Step 1. NH+ forms double bond with 40KN The isotope of potassium used is 40K. 40K is a natural, long-lived radioactive isotope of potassium. The dots represent unpaired electrons, and the numbers represent the number of electrons in the outer shell of each nitrogen. This compound is unbalanced and unstable.
.H+N=NK:
7 8
Step 2. 41Ca+ is added to the original compound, N2KH++ 41Ca+
The isotope of calcium used is 41Ca. 41Ca is a natural, long-lived radioactive isotope of calcium. This compound is balanced, but unstable because 41Ca will naturally decay.
Ca+H+N=NK:
8 8
Step 3. 41Ca+ decays to 40K and H+.
Under normal circumstances, 41Ca usually decays to 41K through electron capture. However, in this case since the cation of 41Ca is being used, only one electron is available in the outer shell of 41Ca+. This electron is strongly attached to the nitrogen, which is now balanced, making it unavailable to the 41Ca+. Therefore, using an electron from an inner shell would result in an undesirable total number of electrons for 41Ca+. For this reason, in this instance, I believe electron capture may not be the best option. However, 41Ca+ still must decay. Other ways of decay, such as neutron activation or beta decay, are either not available or lead to a more unstable state. Therefore, with the energy available from the decay of 41Ca+ and possibly the decay of the current 40K, 41Ca+ removes a proton, creating another 40K, a long-lived radioactive isotope of potassium (even longer-lived than 41Ca) and a free proton (H+). This proton joins the available lone pair, and 40K replaces 41Ca+. This creates the most balanced version of this compound to date. However, it is still unstable because 40K will continually decay.
H+KN=NKH+
8 8
Step 4. The original 40K decays to 40Ca through beta decay
The original 40K has been naturally decaying to 40Ca. However, with the creation of 40Ca, another electron is now available. Therefore, the 40Ca unbalances the formerly balanced compound.
H+KN=NH+ Ca
8 9
Step 5. 40Ca decays to 41Ca+ through neutron activation
The decay of 40Ca to 41Ca is a natural process, under the right conditions, even though 40Ca is stable. Similarly, in this case, the desire to lose the extra electron (created from the decay of 40K to 40Ca) causes 40Ca to use this extra electron, in combination with the available proton (H+), to decay to 41Ca+. This occurs through, what would basically be, neutron activation. This process rebalances the compound, creating essentially the same compound as in step 2.
H+KN=NCa+:
8 8
It also allows for a continuous cycle of steps 2 through 5, creating reflections of itself as it passes through a neutral state.
Step 5 (or 2) > Step 3 > Step 4 > Step 5 (or 2) > Step 3 > Step 4 > back to the beginning
H+KN=NCa+: > H+KN=NKH+ > CaH+N=NKH+ > :Ca+N=NKH+ > H+KN=NKH+ > H+KN=NH+Ca > back to the beginning
+ > N > - > - > N > + > back to the beginning
Below is an image of the above because I couldn't get the spacing right