Zuzana
- 12
- 1
Hi,
I would like to ask, why K-shell electrons coming from the internal conversion are much more frequent than L or M-shell electrons (see Fig). K-shell electrons are more tightly bound than L-shell, I would say that it is easier for gamma particle to kick off less tightly electron, no?
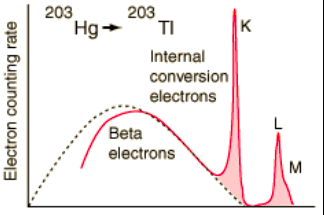
Thank you in advance for the reply.
I would like to ask, why K-shell electrons coming from the internal conversion are much more frequent than L or M-shell electrons (see Fig). K-shell electrons are more tightly bound than L-shell, I would say that it is easier for gamma particle to kick off less tightly electron, no?
Thank you in advance for the reply.