tellmesomething
- 443
- 68
- Homework Statement
- Graph these processes on a PV graph : Isothermal expansion from state A to B, isochoric pressure increment from B to C,
isothermal contraction from C to D, isobaric contraction from D to A.
- Relevant Equations
- !!!
I graphed it similar to this
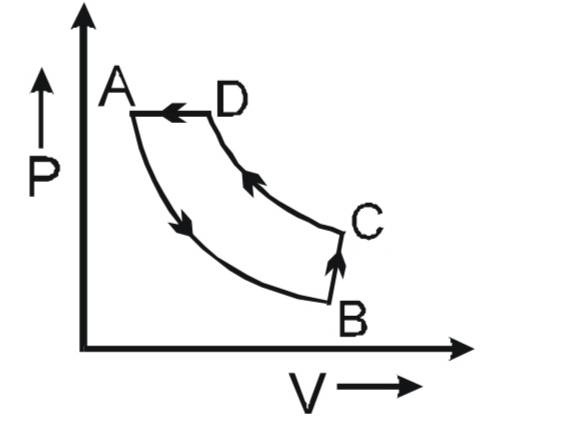
My query is say if the last process wasn't mentioned, I.e the process from A TO D, would the state D have the same pressure as state A then? In thermodynamics for a reversible system we say that if it undergoes a change in pressure volume the exact pressure and volume can he achieved when we reverse it, considering the temperature is constant. But here since we underwent an isochoric process from B to C and then reversed it, would it have ended at the same pressure of A if not for the last condition?
My query is say if the last process wasn't mentioned, I.e the process from A TO D, would the state D have the same pressure as state A then? In thermodynamics for a reversible system we say that if it undergoes a change in pressure volume the exact pressure and volume can he achieved when we reverse it, considering the temperature is constant. But here since we underwent an isochoric process from B to C and then reversed it, would it have ended at the same pressure of A if not for the last condition?
Last edited: