Gigantron
- 11
- 0
"Guess the Mechanism" help! (Organic Chemistry)
After identifying my reactants and products via spectral analysis, I am now tasked with figuring out the mechanism behind converting a diol into a ketone.
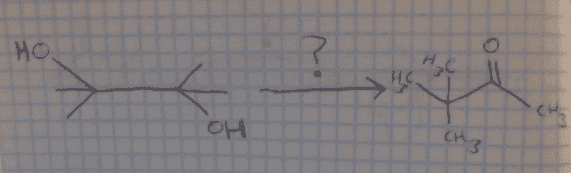
The key to this appears to be tautomerization somewhere in the reactant (whereby the oxygen atoms donate electrons in order to create a double bond), but everything I've tried with that has failed so far. I'm really stuck.
Homework Statement
After identifying my reactants and products via spectral analysis, I am now tasked with figuring out the mechanism behind converting a diol into a ketone.
Homework Equations
The Attempt at a Solution
The key to this appears to be tautomerization somewhere in the reactant (whereby the oxygen atoms donate electrons in order to create a double bond), but everything I've tried with that has failed so far. I'm really stuck.