TheePhysicsStudent
- 21
- 17
- Homework Statement
- I was practising questions from a book printed in the 1980s, and I'm unsure where I went wrong with this Q, the answer is 68.2%
- Relevant Equations
- I dont use equations for chemistry mole calculations, i just think my way through it so I don't know
The question

My Working:
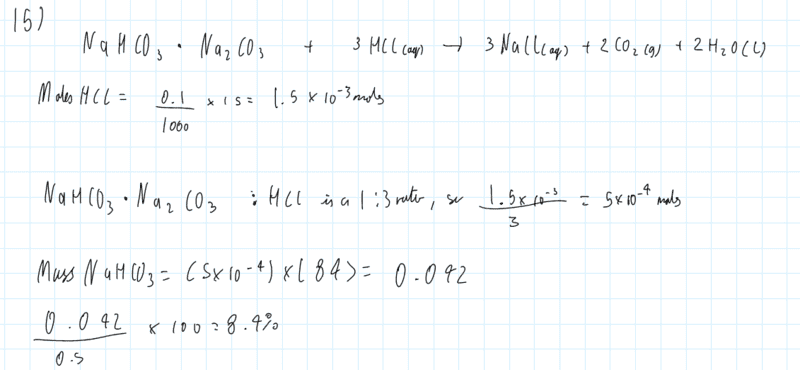
My Working: