Calafalas
- 2
- 0
Hi chemistry friends,
I'm currently working on a reaction to produce the following polymer: Cyclopentadiene functionalized polyketone.
I start with polyketone as a reagent and I should end up with the reaction product together with the solvent (THF) and water with NaOH.
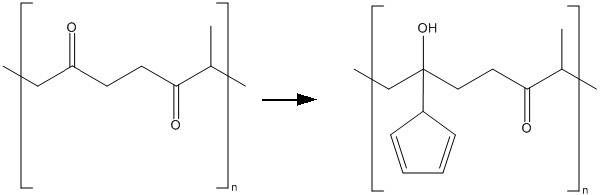
My question is as following: How can I separate my reaction product from the THF/water ?
Do you think that is will be insoluble in THF/water, so I can use simple filtration? Or should I use a different solvent to extract the polymer ?
I'm currently working on a reaction to produce the following polymer: Cyclopentadiene functionalized polyketone.
I start with polyketone as a reagent and I should end up with the reaction product together with the solvent (THF) and water with NaOH.
My question is as following: How can I separate my reaction product from the THF/water ?
Do you think that is will be insoluble in THF/water, so I can use simple filtration? Or should I use a different solvent to extract the polymer ?