Daniel2244
- 125
- 5
Thread moved from the technical forums, so no Homework Template is shown
The enthalpy of formation equation:
ΔHƒ°reaction=∑Δhƒ°(products)-ΣΔHƒ°(reactants)
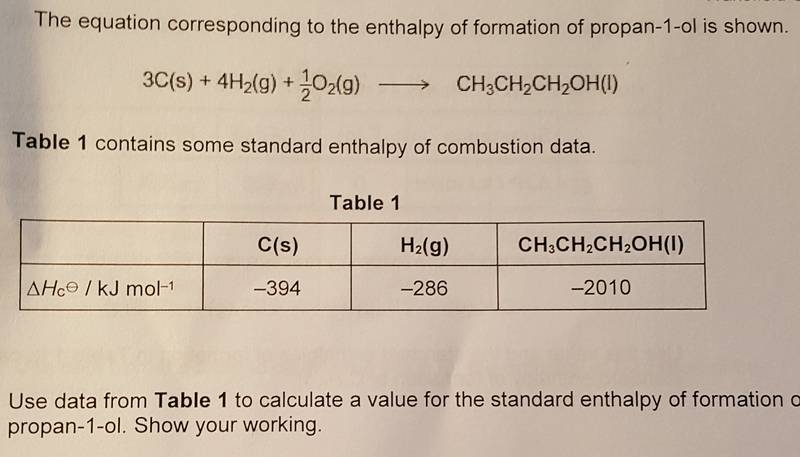
When using the enthalpy of formation equation you get:
-2010-((-394x3)+(-286x4))= 316Kjmol-1
However, this answer is wrong. On the mark scheme, the answer is -316Kjmol-1.
So I used the enthalpy of combustion equation:
ΔHc°reaction=∑Δhc°(reactants)-ΣΔHc°(Products)
((-394x3)+(-286x4))--2010-= -316Kjmol-1
When using the enthalpy of combustion equation I got the correct answer. This is where I get confused as the question is asking for the enthalpy of formation of propan-1-ol, so shouldn't I use the enthalpy of formation equation? Or do I have to use the enthalpy of combustion equation as I am using combustion data?
ΔHƒ°reaction=∑Δhƒ°(products)-ΣΔHƒ°(reactants)
When using the enthalpy of formation equation you get:
-2010-((-394x3)+(-286x4))= 316Kjmol-1
However, this answer is wrong. On the mark scheme, the answer is -316Kjmol-1.
So I used the enthalpy of combustion equation:
ΔHc°reaction=∑Δhc°(reactants)-ΣΔHc°(Products)
((-394x3)+(-286x4))--2010-= -316Kjmol-1
When using the enthalpy of combustion equation I got the correct answer. This is where I get confused as the question is asking for the enthalpy of formation of propan-1-ol, so shouldn't I use the enthalpy of formation equation? Or do I have to use the enthalpy of combustion equation as I am using combustion data?

