WMDhamnekar
MHB
- 376
- 28
Robert Boyle's law states that at constant temperature, the pressure of fixed amount ( i-e number of moles n) of gas varies inversely with its volume. Mathematically, it can be written as $p ∝ \frac1V $(at constant T and n) $\Rightarrow p = k_1 \times \frac1V $ where $k_1$is a proportionality constant.
The value of constant $k_1$ depends upon the amount of the gas, temperature of the gas and the units in which p and V are expressed. $p \times V= k_1$
If a fixed amount of gas at constant temperature T occupying volume $V_1$ at pressure $p_1$ undergoes expansion, so that volume becomes $V_2$ and pressure becomes $p_2,$ then according to Boyle’s law : $p_1 \times V_1 = p_2 \times V_2=$ constant $\Rightarrow \frac{p_1}{p_2} = \frac{V_2}{V_1}.$
It should be noted that volume V of the gas doubles, if pressure is halved.
The following table 5.1 gives effect of pressure on volume of 0.09 mol of CO₂ at 300 K. but i didn't understand these calculated values given in the second column. I also didn't understand the meanings of headings given to each column. If any member can explain me how the values in the second column is computed, may answer to this question.
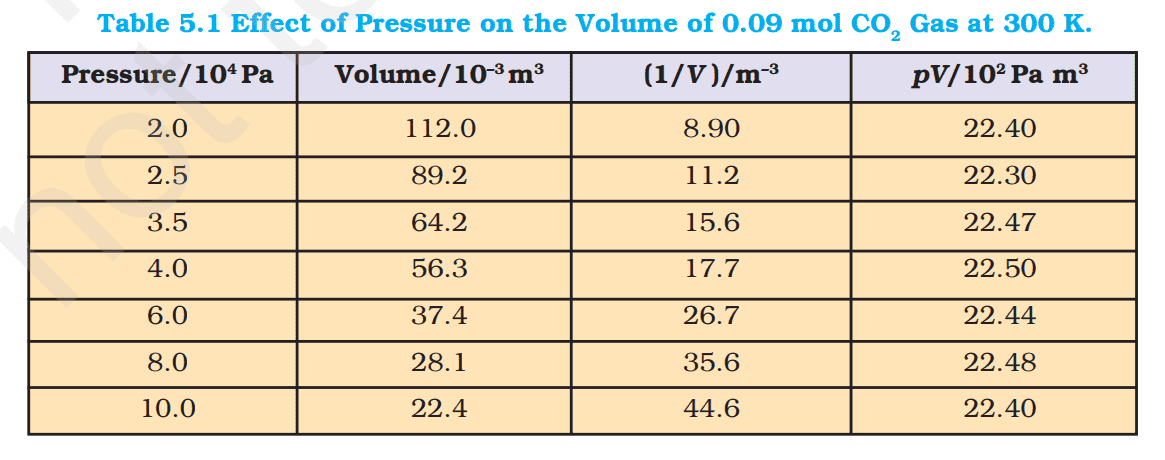 My understanding:
My understanding:
$V= \frac{nRT}{p}\tag {1}$ where n, R, T, p are constants. n stands for number of moles, R is gas constant, T is temperature and p is pressure.
Putting the given values in this equation (1),we get 11.2 liters =$\frac {0.09 mol \times 8,314 J k^{-1} mol^{-1}\times 300 K }{20000 Pa}$
But in the second column, it is $112 \times 10^{-3} m^3= 112 $ liters . How is that? Where i am wrong?Can we compute the volume of $CO_2$ in another way? For example, by using this known information that one mole of $CO_2$ molecules features a volume of 22.414 liters at standard T and p. So, 0.09 mol of $CO_2$ features a volume of $0.09 \times 22.414= 2.01726 $ liters at STP.
The value of constant $k_1$ depends upon the amount of the gas, temperature of the gas and the units in which p and V are expressed. $p \times V= k_1$
If a fixed amount of gas at constant temperature T occupying volume $V_1$ at pressure $p_1$ undergoes expansion, so that volume becomes $V_2$ and pressure becomes $p_2,$ then according to Boyle’s law : $p_1 \times V_1 = p_2 \times V_2=$ constant $\Rightarrow \frac{p_1}{p_2} = \frac{V_2}{V_1}.$
It should be noted that volume V of the gas doubles, if pressure is halved.
The following table 5.1 gives effect of pressure on volume of 0.09 mol of CO₂ at 300 K. but i didn't understand these calculated values given in the second column. I also didn't understand the meanings of headings given to each column. If any member can explain me how the values in the second column is computed, may answer to this question.
$V= \frac{nRT}{p}\tag {1}$ where n, R, T, p are constants. n stands for number of moles, R is gas constant, T is temperature and p is pressure.
Putting the given values in this equation (1),we get 11.2 liters =$\frac {0.09 mol \times 8,314 J k^{-1} mol^{-1}\times 300 K }{20000 Pa}$
But in the second column, it is $112 \times 10^{-3} m^3= 112 $ liters . How is that? Where i am wrong?Can we compute the volume of $CO_2$ in another way? For example, by using this known information that one mole of $CO_2$ molecules features a volume of 22.414 liters at standard T and p. So, 0.09 mol of $CO_2$ features a volume of $0.09 \times 22.414= 2.01726 $ liters at STP.
Last edited: