A13235378
- 50
- 10
Thread moved from the technical forums to the schoolwork forums
Summary:: Isomery
My attemption: (I found 5)
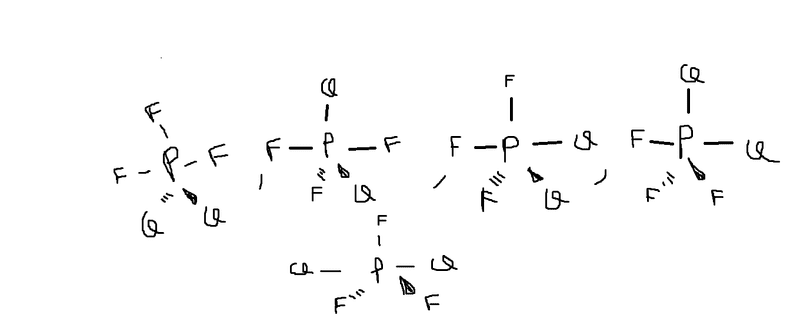
But the answer is 3 forms, where I missing?
My attemption: (I found 5)
But the answer is 3 forms, where I missing?