Spathi
Gold Member
- 102
- 10
I have a question relating this molecule (bodipy) :
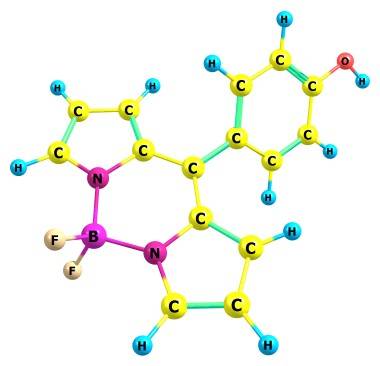
How do modern empirical models in chemistry explain the B-N bonds in this compound? Maybe we should consider this bond as having bond order = 0.5?
The same story with metalloporphyrins.
How do modern empirical models in chemistry explain the B-N bonds in this compound? Maybe we should consider this bond as having bond order = 0.5?
The same story with metalloporphyrins.