i_love_science
- 80
- 2
- Homework Statement
- question attached below
- Relevant Equations
- resonance structures
Question:
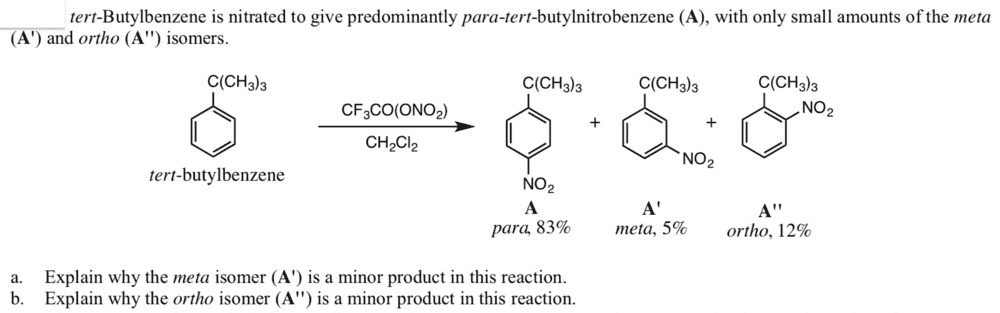
Answer:
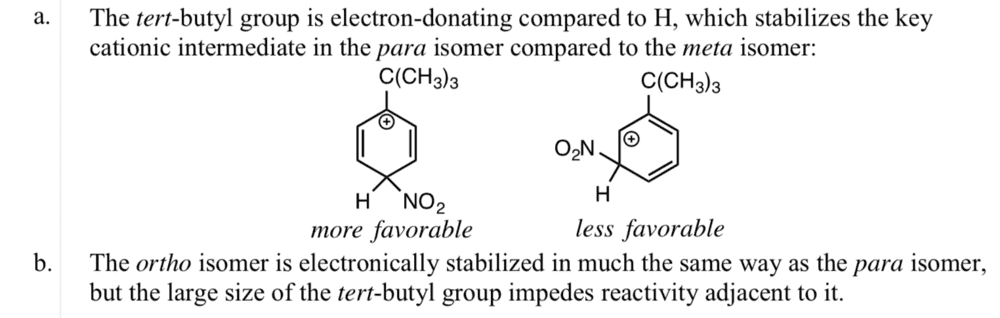
I don't understand how the para and meta resonance structures were created (what the electron transfer was). Also, why is the ortho isomer stabilized in the same way as the para isomer? Thanks.
Answer:
I don't understand how the para and meta resonance structures were created (what the electron transfer was). Also, why is the ortho isomer stabilized in the same way as the para isomer? Thanks.