i_love_science
- 80
- 2
- Homework Statement
- see below
- Relevant Equations
- titration curve
A weak base is titrated with a strong acid.
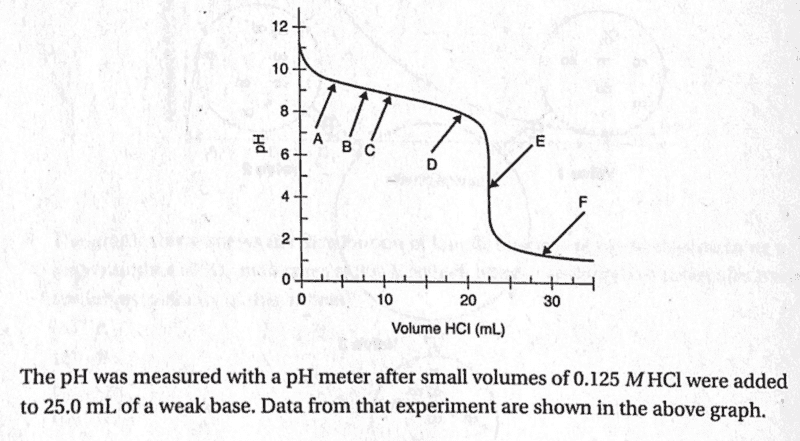

I think that both B and C are correct. The endpoint volume will be lower than the actual equivalence point volume. When the student mistakenly assumes the endpoint volume is the equivalence point volume, the number of moles of strong acid is seen as equal to the number of moles of weak base. The calculated number of moles of strong acid would be lower (because of the lower volume), and the molarity of the weak base would also be lower.
The answer says only B is correct. Could anyone explain why, and where I went wrong? Thanks.
I think that both B and C are correct. The endpoint volume will be lower than the actual equivalence point volume. When the student mistakenly assumes the endpoint volume is the equivalence point volume, the number of moles of strong acid is seen as equal to the number of moles of weak base. The calculated number of moles of strong acid would be lower (because of the lower volume), and the molarity of the weak base would also be lower.
The answer says only B is correct. Could anyone explain why, and where I went wrong? Thanks.