- #1
Goodver
- 102
- 1
I am confused with the classical approach of usage of a "transition dipole oscillation" in order to explain the broadening of spectrum of emission between energy levels.
1. If I understand it correctly then emission of photon is due to oscillation of a dipole consisting of an electron-proton pair, right? In this case, electrons just oscillate on orbitals but do not change their orbitals? Or electrons also jump to the higher and lower orbitals? If they change, then electron oscillates on its way from one orbital to another?
2. Lifetime defined as a time until electron oscillation gets damped, but electron stay on its orbital or time takes for electron to transit from one orbital to another? What determines the different lifetimes for different atoms?
3. Why broadening of a spectrum can not be explain with a solid state physics concept of Kronig-Penney model, where we have bands instead of level in a multi atomic system?
4. Why dipole can oscillates only with a frequency corresponding to the energy difference between energy levels?
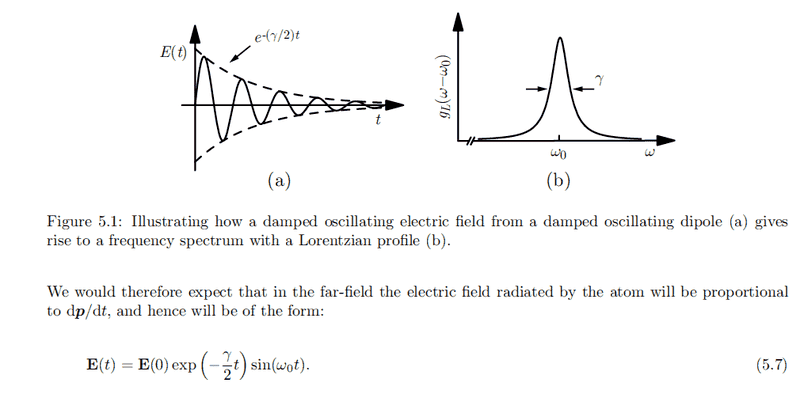
Picture taken from this thread:
Thank you.
1. If I understand it correctly then emission of photon is due to oscillation of a dipole consisting of an electron-proton pair, right? In this case, electrons just oscillate on orbitals but do not change their orbitals? Or electrons also jump to the higher and lower orbitals? If they change, then electron oscillates on its way from one orbital to another?
2. Lifetime defined as a time until electron oscillation gets damped, but electron stay on its orbital or time takes for electron to transit from one orbital to another? What determines the different lifetimes for different atoms?
3. Why broadening of a spectrum can not be explain with a solid state physics concept of Kronig-Penney model, where we have bands instead of level in a multi atomic system?
4. Why dipole can oscillates only with a frequency corresponding to the energy difference between energy levels?
Picture taken from this thread:
Thank you.
Last edited: