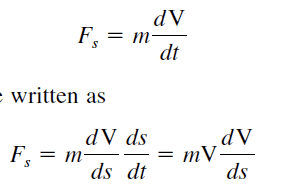- #1
th3plan
- 93
- 0
I am reading a thermodynamics book. I am confused on how they say use the chain rule Here. it makes no sense to me how they go from dV/dt to (dV/ds)(ds/dt) . I know how the chain rule works ,just don't know where they got these values