Dario56
- 289
- 48
I have two gas tanks of nitrogen and oxygen. Mixture of gases is created and used in the system of interest.
Let's say I want to create an atmospheric conditions in my system, mass ratio of oxygen to nitrogen is 0.3 and total pressure of 1 bar (oxygen partial pressure 0.21 bar). Therefore, mass flow ratio of the gases needs to be 0.3 to create a gas mixture of atmospheric composition. Gas tanks are equipped with volumetric flowmeters. This isn't a problem as mass and volumetric flowrates can be easily related by the ideal gas law. I'm planning using air at atmospheric pressure and temperatures 500-800 ##^\circ$## which I think justifies ideal gas law.
What I'm not clear about is when the tanks are opened and the flowrates are specified, what will determine the pressure in the system? This is important as I need to know and change the oxygen partial pressure during this experiment (electrical conductivity relaxation). The papers I've read about this method mention that change in oxygen partial pressure is induced by the change in the flowrate ratio (Dalton's law). This assumes that total pressure in the system is constant and therefore independent of the gas flowrates.
In another words, when the change in oxygen partial pressure is increased, why would nitrogen partial pressure decrease in this setup (or vice versa)? What mechanism is keeping the total pressure constant?
Schematic is provided to give a better insight into the process:
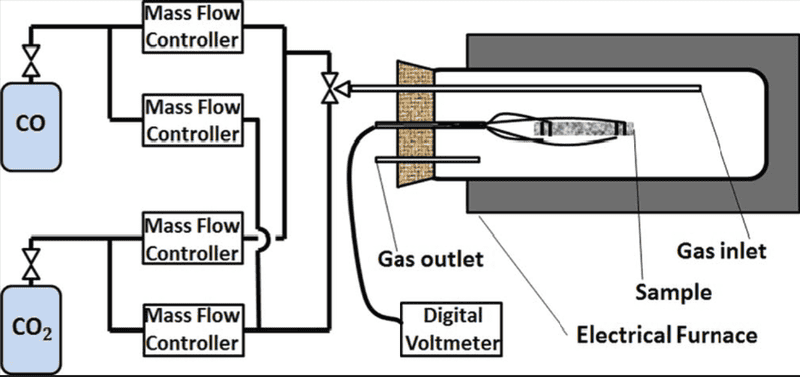
Let's say I want to create an atmospheric conditions in my system, mass ratio of oxygen to nitrogen is 0.3 and total pressure of 1 bar (oxygen partial pressure 0.21 bar). Therefore, mass flow ratio of the gases needs to be 0.3 to create a gas mixture of atmospheric composition. Gas tanks are equipped with volumetric flowmeters. This isn't a problem as mass and volumetric flowrates can be easily related by the ideal gas law. I'm planning using air at atmospheric pressure and temperatures 500-800 ##^\circ$## which I think justifies ideal gas law.
What I'm not clear about is when the tanks are opened and the flowrates are specified, what will determine the pressure in the system? This is important as I need to know and change the oxygen partial pressure during this experiment (electrical conductivity relaxation). The papers I've read about this method mention that change in oxygen partial pressure is induced by the change in the flowrate ratio (Dalton's law). This assumes that total pressure in the system is constant and therefore independent of the gas flowrates.
In another words, when the change in oxygen partial pressure is increased, why would nitrogen partial pressure decrease in this setup (or vice versa)? What mechanism is keeping the total pressure constant?
Schematic is provided to give a better insight into the process:
Last edited: